1.7: Connecting the van der Waals and the viral equations: the Boyle temperature - Chemistry LibreTexts

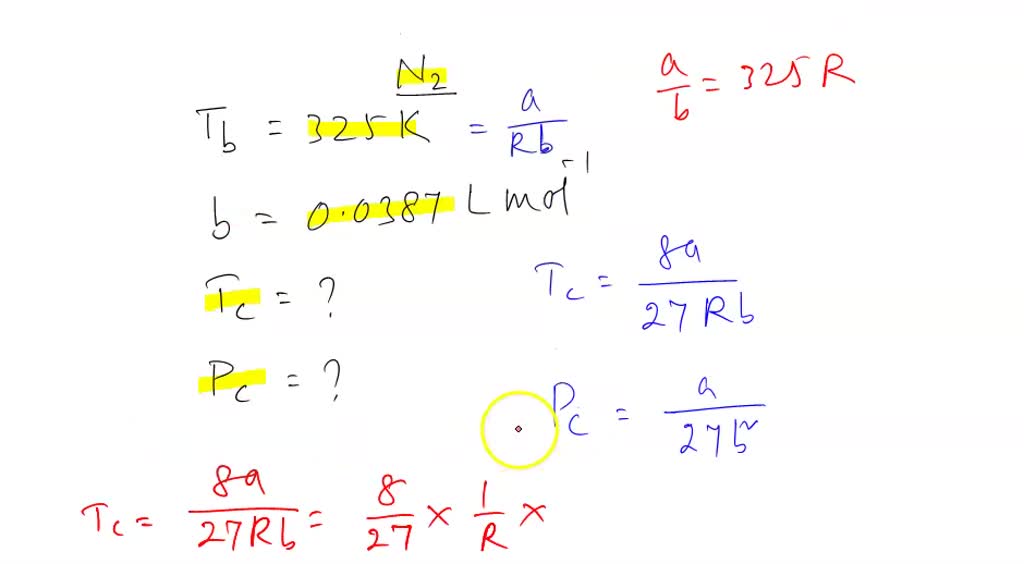
SOLVED: The Boyle temperature of N2(g) is 325 K and its van der Waals b parameter is 0.0387 L mol-1 . Calculate its critical temperature (K) and critical pressure (bar).

Van der Waals Equation Practice Problems

For a van der Waal's gas, determine Boyle Temperature. [ given mathrm{a}=4.5 mathrm{atm} mathrm{L}^2mathrm{mol}^{-2}, mathrm{b}=0.9 mathrm{L} mathrm{mol}^{-1} and R=0.082 mathrm{L} mathrm{atm} mathrm{K}^{-1} mathrm{mol}^{-1}].609.8K6.09K273K60.98K

Boyle Temperature for Van der Waals, Berthelot and Dieterici, Unit 2, BPC Class
How do Van der Waals constants a and b depend on temperature, pressure and volume? - Quora

Van der Waals Equation Practice Problems
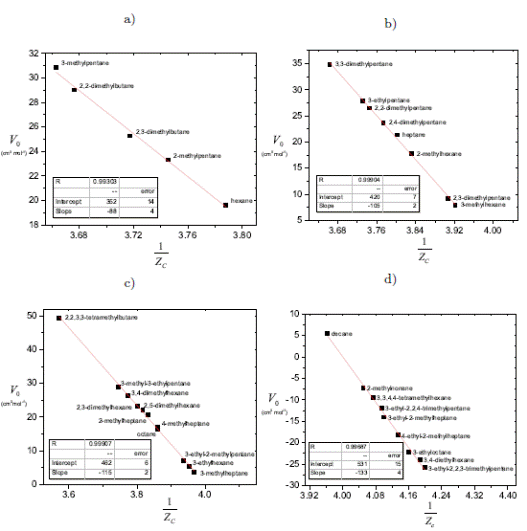
Critical Constants Correlation from van der Waals Equation

1.7: Real Gases - Chemistry LibreTexts

Van der Waals Equation Practice Problems

Full PDF, PDF, Cell Nucleus











