Palette Life Sciences Announces FDA 510(k) Clearance for Barrigel

Groundbreaking technology introduces increased control to achieve optimal coverage proven to significantly reduce the risk of toxicity to the rectum SANTA BARBARA, CALIF. / STOCKHOLM, SWEDEN – June 9, 2022— Palette Life Sciences, a fully-integrated global life sciences company dedicated to improving patient outcomes, today announced U.S. Food and Drug Administration (FDA) 510(k) clearance of […]

Palette Life Sciences Enters Into Definitive Agreement with Teleflex Incorporated
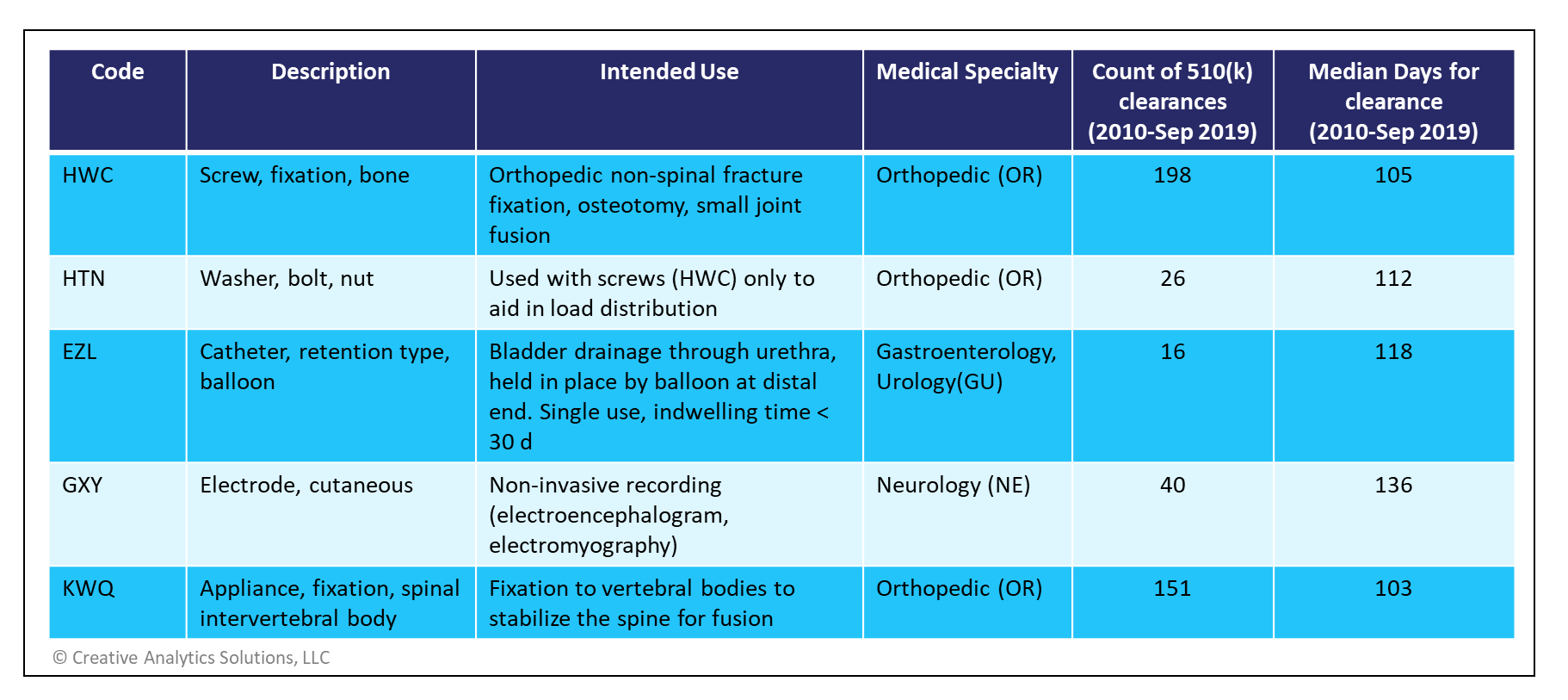
A Pathway to Faster FDA Approvals — Exeed

Marianna Hawkins posted on LinkedIn

Per-Olov Wedin posted on LinkedIn
SpaceOAR - Augmenix, Boston Scientific, and Conflicts of Interest, Page 4
Control Matters Barrigel for Healthcare Providers

CAROL THRONDSON on LinkedIn: Barrigel

Vinny Devany on LinkedIn: Hyaluronic Acid Spacer for Hypofractionated Prostate Radiation Therapy

A Pathway to Faster FDA Approvals — Exeed





:upscale()/2023/10/23/710/n/1922441/dd7503495b9029c7_stanley-quench-2.0-tumbler-crop.jpg)

