At low pressure, the van der waal's equation is written as (P+ a/V
At low pressure, the van der waal's equation is written as (P+ a/V^2)V=RT . Then compressibility factor is then equal to :
At low pressure- the van der waal-s equation is written as -P- a-V-2-V-RT - Then compressibility factor is then equal to

Van der Waals Equation: Definition, Derivations, and Examples

toppr-doubts-media.s3.aws.com/images/1336646
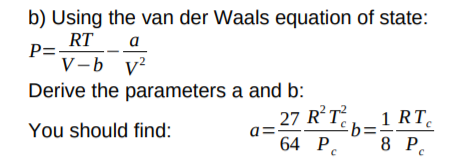
Answered: b) Using the van der Waals equation of…
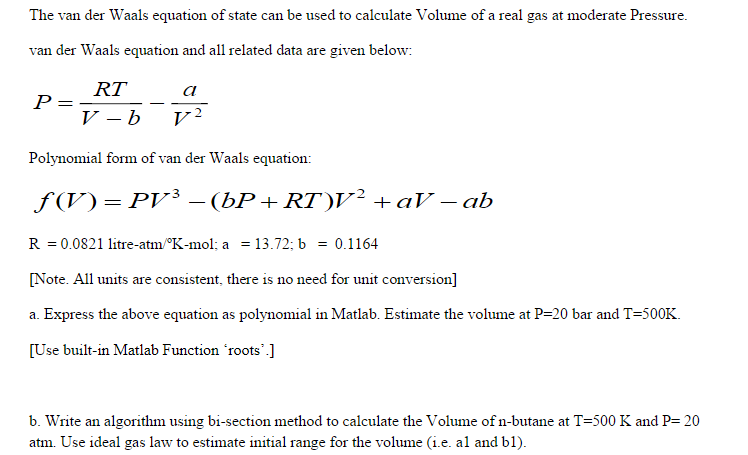
Solved The van der Waals equation of state can be used to
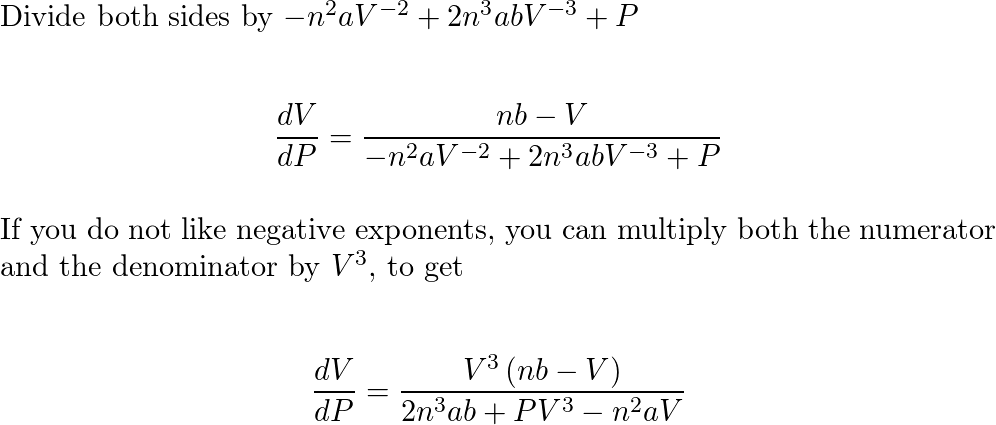
The van der Waals equation for n moles of a gas is $$ (P+n

Bengali] At a low pressure, the van der waals equation reduces to (P+
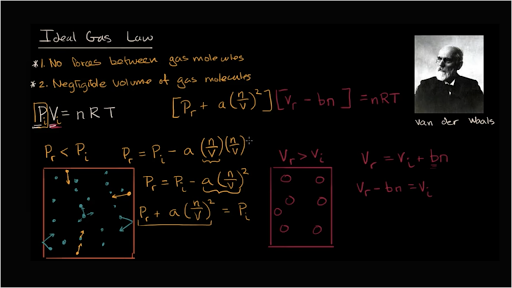
The van der Waals equation (video)

Why do we use the ideal gas equation when instead van der Waals

Van Der Waals Equation - an overview
✓ Solved: van der Waals Equation Calculate the pressure of water

At high temperature and low pressure, the van der Waals' equation
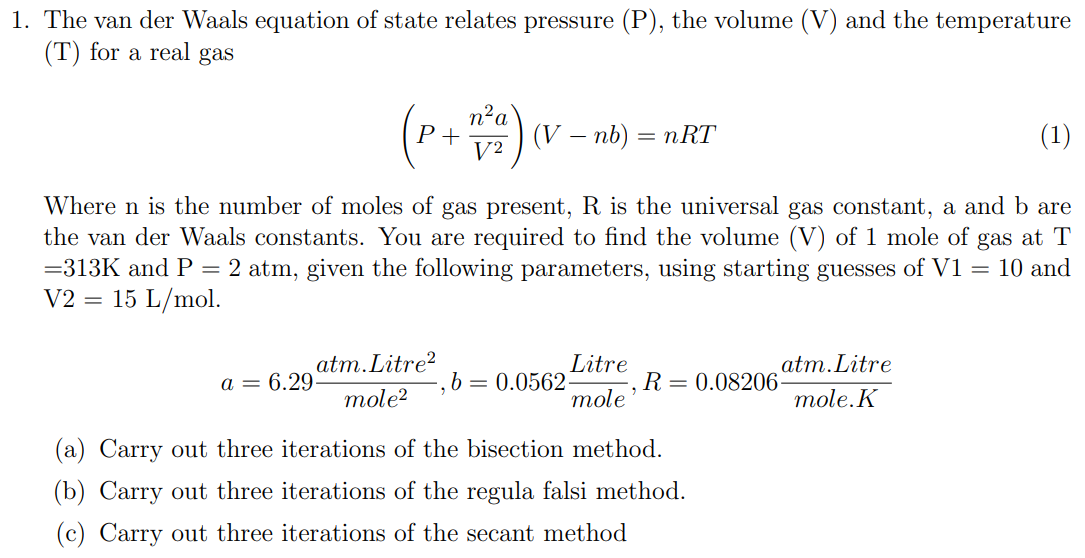
Solved The van der Waals equation of state relates pressure

At low pressures For 1 mole, the van der Waals equation is written