The Cottrell Experiment and Diffusion Limitation 3/3 - Electrochemical Double Layer - PalmSens
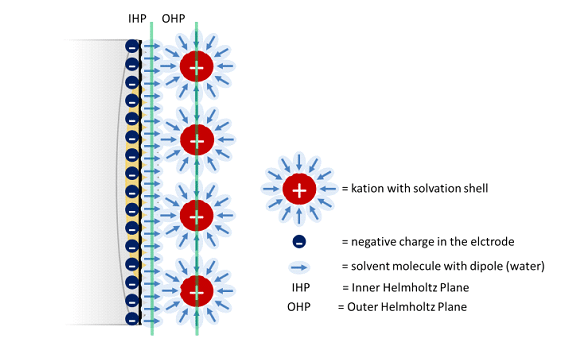
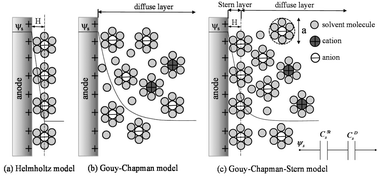
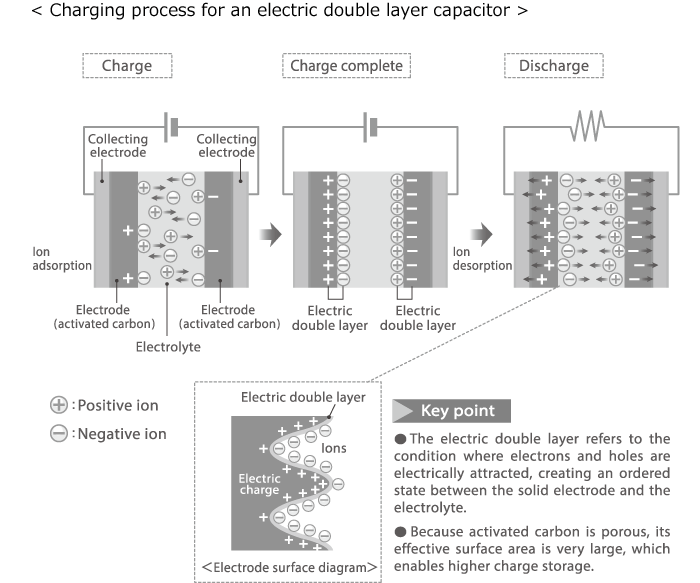
In this chapter the electrochemical double layer and its features are discussed. The electrochemical double layer acts as a capacitor and every change in the potential of the electrode will induce a capacitive charging current that is caused by physics not by a chemical reaction. This current decays exponentially.

More Accurate Measurement of Return Peak Current in Cyclic

Alternative representation of the Cottrell diffusion according to

Emergence of a Stern Layer from the Incorporation of Hydration

Electric Double Layer - an overview
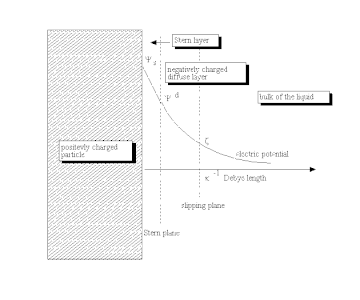
Double layer (surface science) - Wikipedia

Cottrell's equation revisited: an intuitive, but unreliable, novel

Electrochemical Double Layer - an overview

a) Structure of electric double layer, with the corresponding

More Accurate Measurement of Return Peak Current in Cyclic
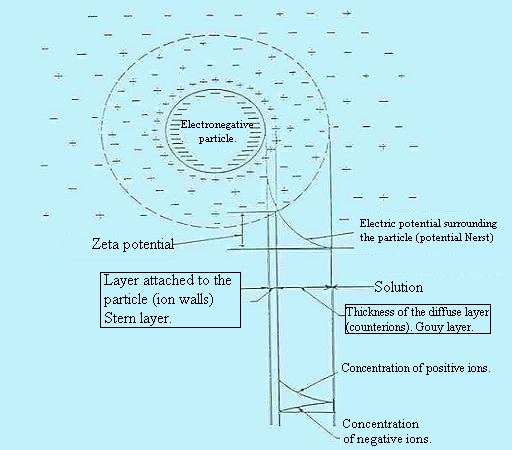
Coagulation

Schematic representation of the double layer and potential drop

PDF) Finite Heterogeneous Rate Constants for the Electrochemical

Cottrell's equation revisited: an intuitive, but unreliable, novel

A new approach to characterising the porosity of particle modified
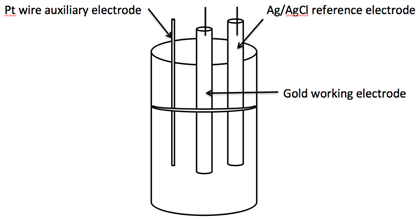
Introduction to Electrochemistry