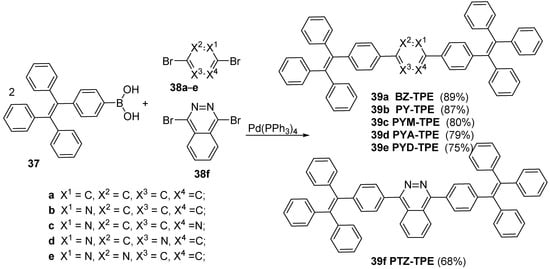
The synthesis of fluorescent azaheterocycles continues to arouse strong interest due to their great potential for application as sensors and biosensors, luminophores on in the construction of Organic Light-Emitting Diode (OLED) devices, laser and other semiconductor devices, as well as to their potential biological properties as antimicrobial, antifungal, anticancer, antituberculosis antioxidant and anti-HIV agents. The advantages of the azaheterocyclic fluorophores, such as small size, enriched photostability, a wide and tunable spectral range, and, frequently, high brightness, are the reason why these fluorophores are preferred and used in various medical application. Probe structure can be modified to adjust excitation and emission wavelengths, target-binding affinity, chemical reactivity, and subcellular localization.

Total Synthesis of C60

Preparation of cis-Norbornene-5,6-endo-dicarboxylic Anhydride via the Stereoselective Diels-Alder Reaction of Cyclopentadiene and Maleic Anhydride

Crystals, Free Full-Text
Heterocyclic Chemistry
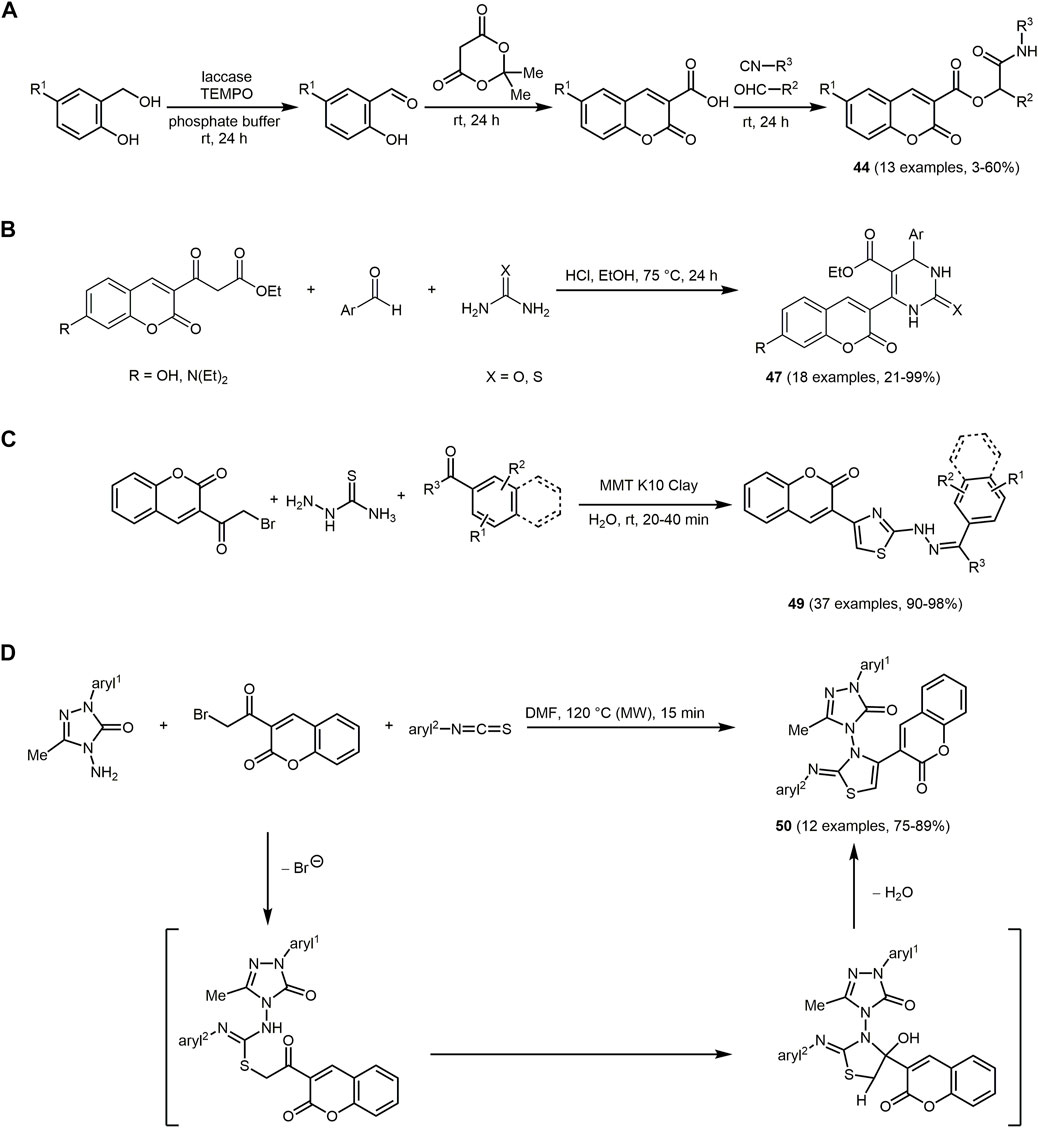
Frontiers Multicomponent synthesis of chromophores – The one-pot approach to functional π-systems
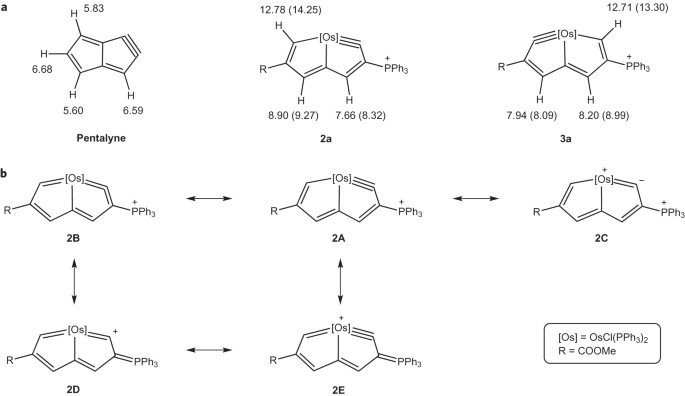
Stabilization of anti-aromatic and strained five-membered rings with a transition metal

Generation and exploration of new classes of antitubercular agents

Molecules, Free Full-Text

PDF) A Review on the Synthesis of Fluorescent Five- and Six-Membered Ring Azaheterocycles

The synthesis of azasteroid derivatives under US irradiation and

Results of the in vitro growth inhibition (GI%) caused by
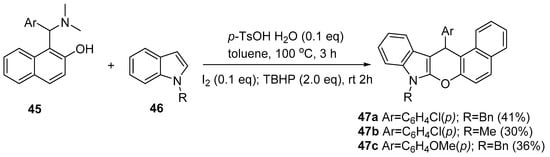
Synthesis of Fluorescent Five- and Six-Membered Ring Azaheterocycles
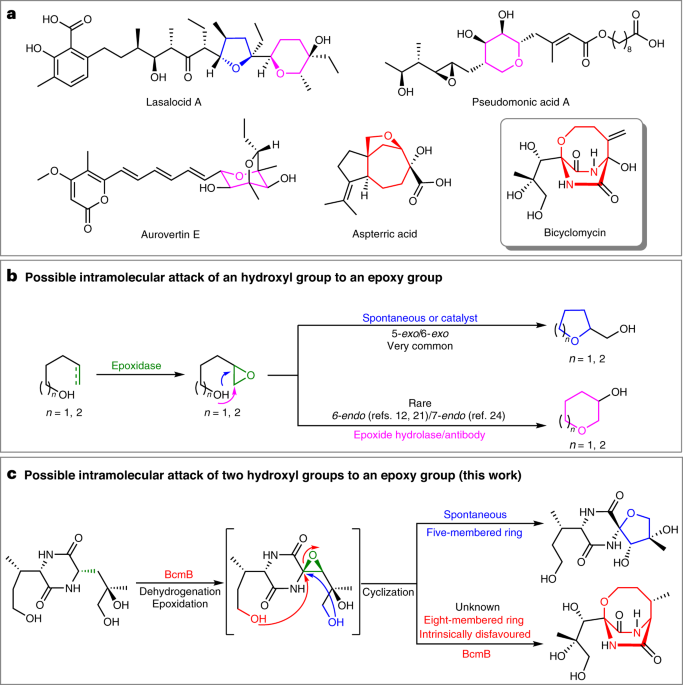
Enzymatic catalysis favours eight-membered over five-membered ring closure in bicyclomycin biosynthesis