24. Assertion :In B2H6, the terminal B H bonds are shorter, than the B H bridge bonds Reason: The terminal B H bond order is greater than that of the B H bridge bond
24- Assertion-In B2H6- the terminal B-H bonds are shorter- than the B-H bridge bonds Reason- The terminal B-H bond order is greater than that of the B-H bridge bond

1 M3 2 Chemical Bonding, PDF, Ionic Bonding

1 M3 2 Chemical Bonding, PDF, Ionic Bonding

The correct statement(s) regarding diborane (B_2H_6) is/are : (a) Maximum six hydrogen atoms can

All the B - H bonds in B_2H_6 are equivalent.truefalse

Which one of the following statements is not true regarding diborane?It has two bridging hydrogens and four perpendicular to the rest.The bridging hydrogens are in a plane perpendicular to the boron atom

The Source Function Descriptor as a Tool to Extract Chemical Information from Theoretical and Experimental Electron Densities

Identify correct order of bond angles (A) C120 > F20 and F20 AsBrz > AsCl3 (C) NO > NOZ vdrogen of B2H6 and Hy is the bridging (D) HBH, >H.BH,; where H

The Source Function Descriptor as a Tool to Extract Chemical Information from Theoretical and Experimental Electron Densities

Formation and Reactivity of Electron‐Precise B−B Single and Multiple Bonds - Arrowsmith - 2017 - Angewandte Chemie International Edition - Wiley Online Library
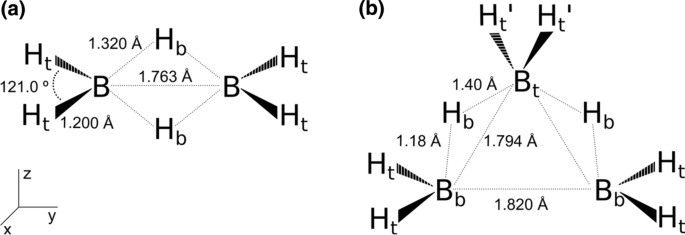
Three-center two-electron bonds in the boranes B2H6 and B3H8− from the quantum interference perspective

The correct statement about B2H6 is
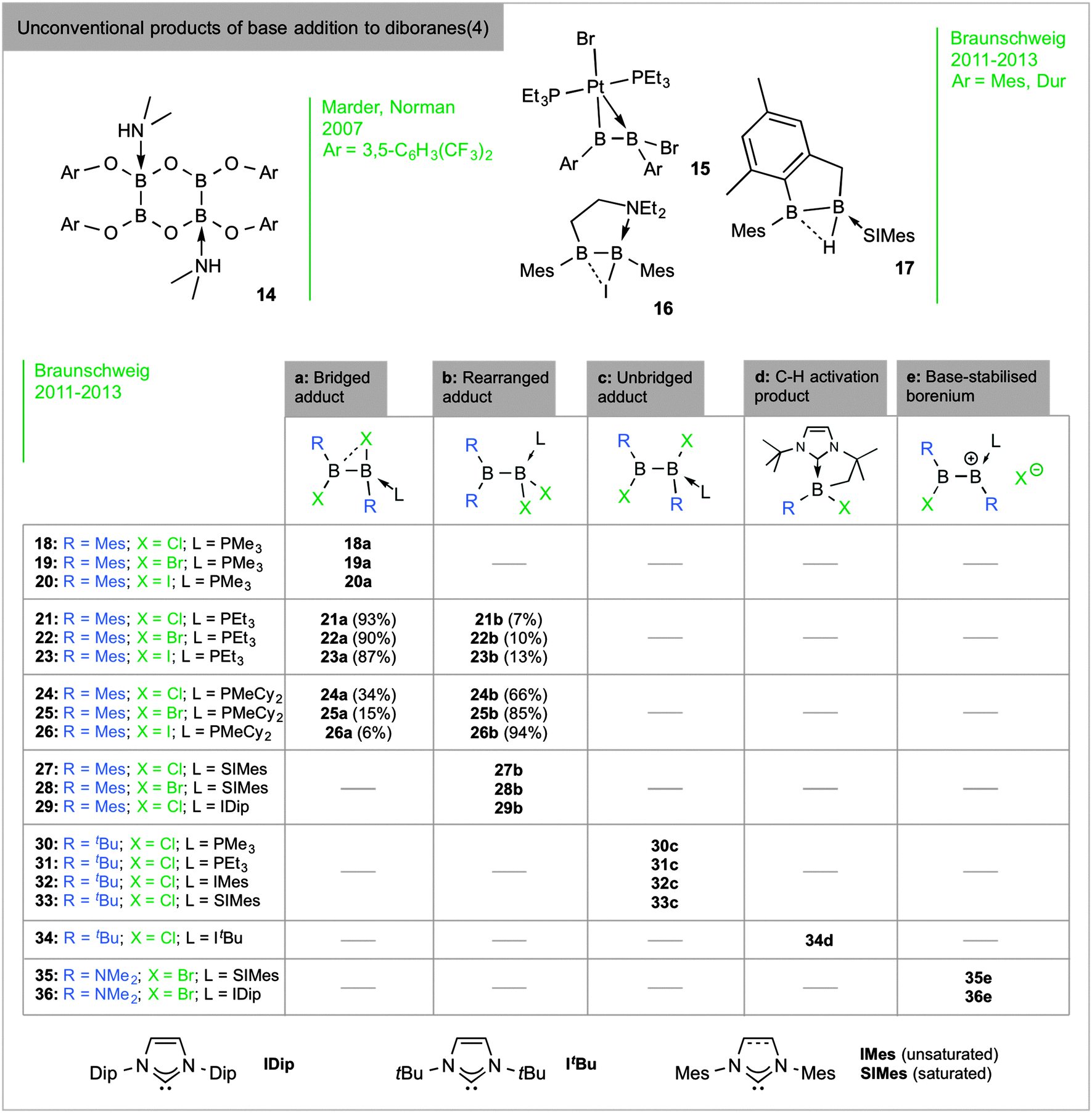
sp 2 –sp 3 diboranes: astounding structural variability and mild sources of nucleophilic boron for organic synthesis - Chemical Communications (RSC Publishing) DOI:10.1039/C5CC02316E

Regeneration of ammonia borane by reduction of polyborazylene (PB) by











