HumanOptics' CustomFlex Artificial Iris Approved as Breakthrough

The first prosthetic iris has been approved by the FDA to treat patients whose eyes have been damaged due to a congenital condition or traumatic injury. HumanOptics’ CustomFlex Artificial Iris can be implanted into a patient to control the amount of light that enters the eye. A rare genetic disorder known as congenital aniridia is […]

mplantation of a custom-made artificial iris (HumanOptics) during

Can HumanOptics change “Human optics history” with its artificial iris?
The 18 most innovative medical devices of 2021 - Page 10 of 19 - Medical Design and Outsourcing
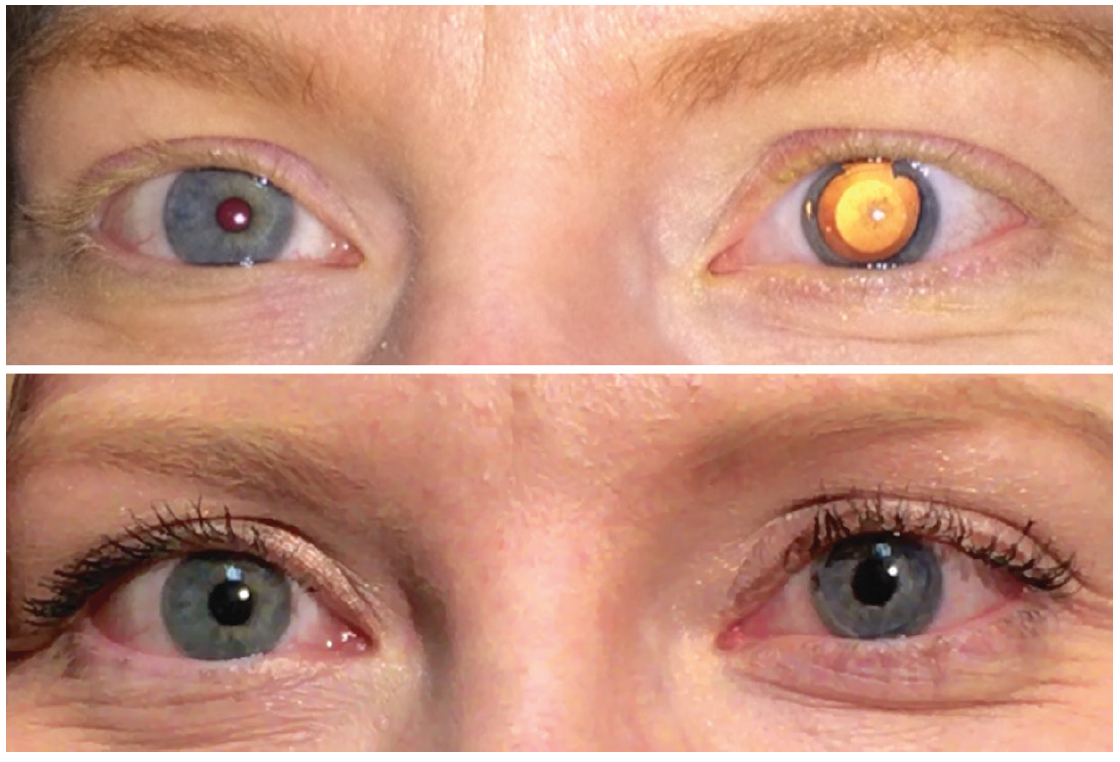
CRSToday A Custom Iris Prosthesis Finally Gains FDA Approval

Saying aye to the artificial iris
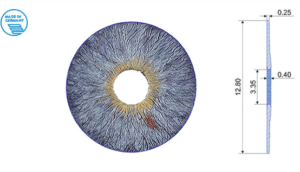
The ARTIFICIALIRIS for your patients with aniridia

Matt Hirabayashi, MD on X: The Silicone HumanOptics Customflex® Artificial Iris was FDA approved in 2018 for medical or aesthetic reconstruction of eyes with complete or partial aniridia. It's the first of
An Insight To Artificial Iris Implants

HumanOptics' CustomFlex Artificial Iris Approved as Breakthrough Device - Xtalks

CUSTOMFLEX® ARTIFICIALIRIS Receives Transitional Pass-through Payment Status from CMS — VEO Ophthalmics
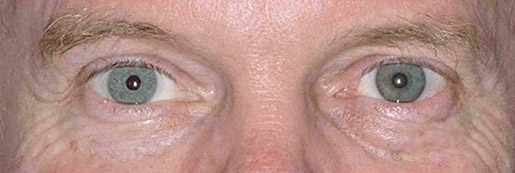
CFAI Patient Page — VEO Ophthalmics

FDA clears first artificial iris from HumanOptics - MassDevice
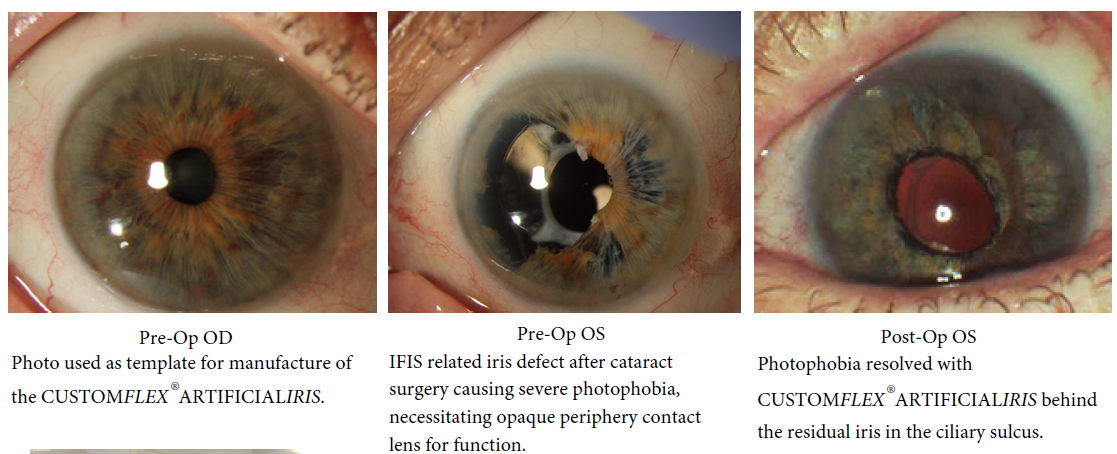
The Artificial Iris: Technically Challenging and Unusually Rewarding - American Academy of Ophthalmology

The ARTIFICIALIRIS for your patients with aniridia







