physical chemistry - Is the compressibility factor smaller or

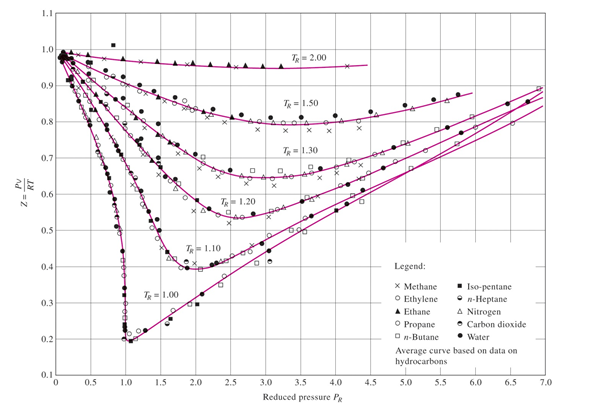
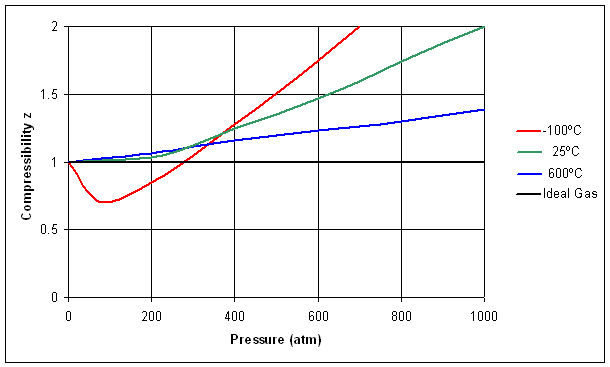
The compressibility factor of a gas is defined as $Z = pV/(nRT)$. If attractive intermolecular forces dominate then $Z$ tends to be smaller than 1, and vice versa if repulsive forces dominate. In

Compressibility factor - Wikipedia

Compressibility Factor Z Important Concepts and Tips for JEE Main
Gas Laws – First Year General Chemistry

Experiments. - ppt video online download

physical chemistry - Why do some gases have lower value of Z for a

where Z is the compressibility factor that

Compressibility factor - Wikipedia
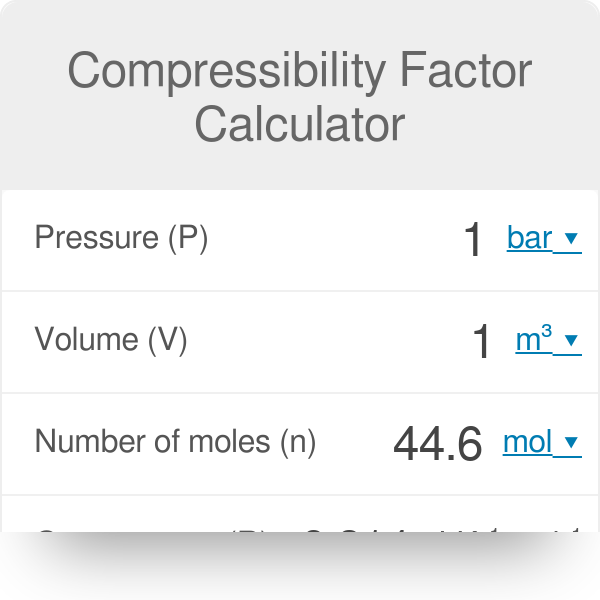
Compressibility Factor Calculator
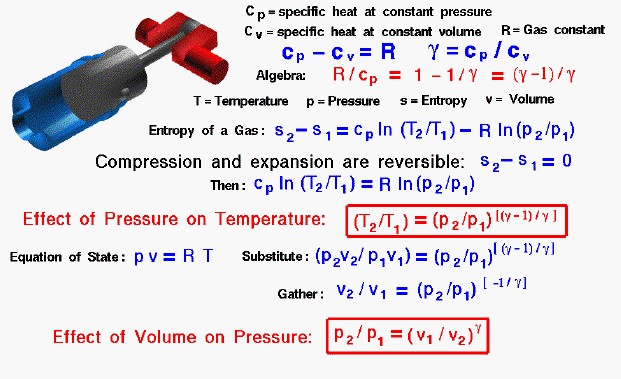
Compression and Expansion, Glenn Research Center

3.2 Real gas and compressibility factor – Introduction to

Real Gases Introductory Chemistry

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks