
pi=CRT" (C = molar concentration)" (pi(1))/(pi(2))=(C(1))/(C(2))," "(4.98)/(1.52)=(36//180)/(C(2))" or "C(2)=(36)/(180)xx(1.52)/(4.98)="0.061 M"

At 300 K,36 g of glucose present per litre in its solution has an osmotic..
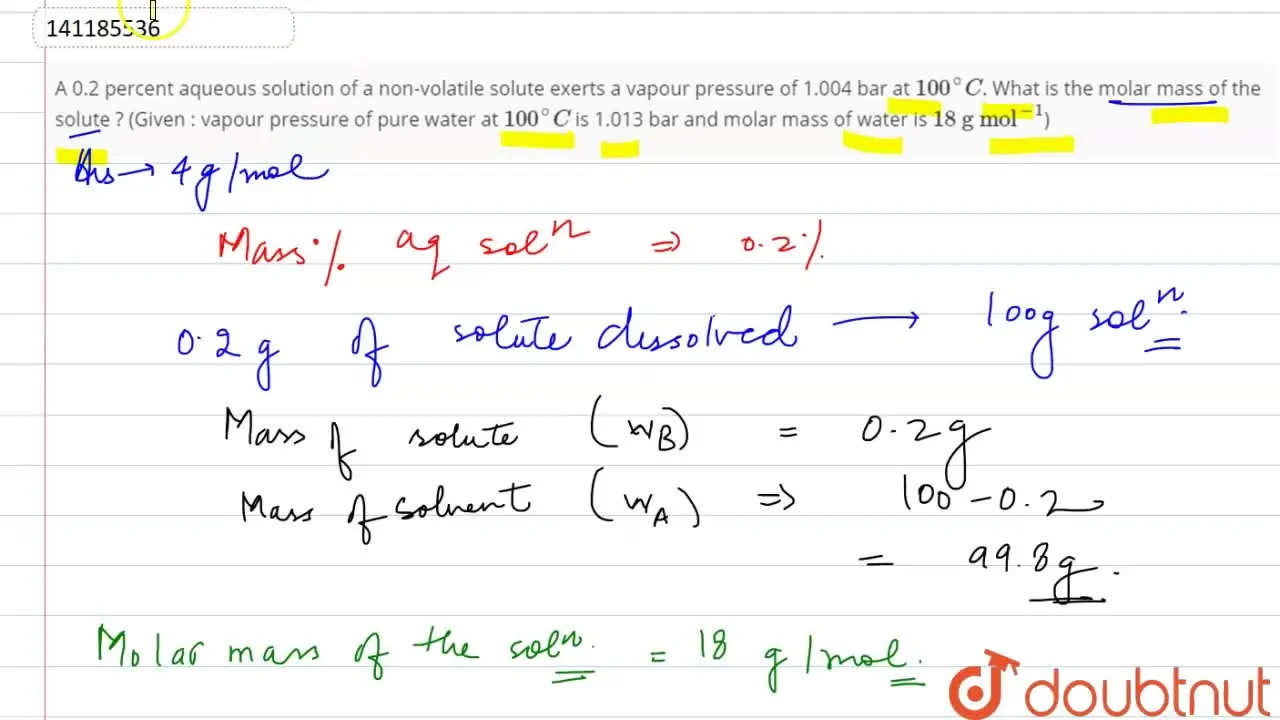
A 0.2 percent aqueous solution of a non-volatile solute exerts a vapou
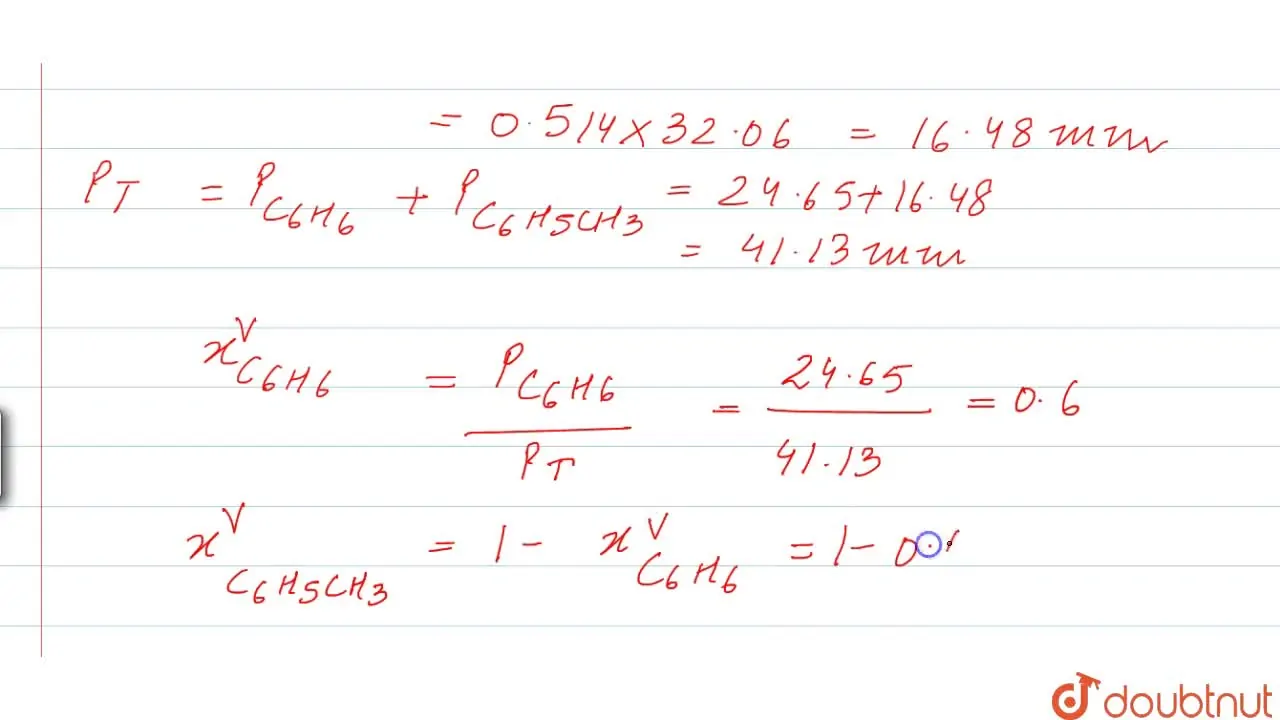
Benzene and toluene form ideal solution over the entire range of comp
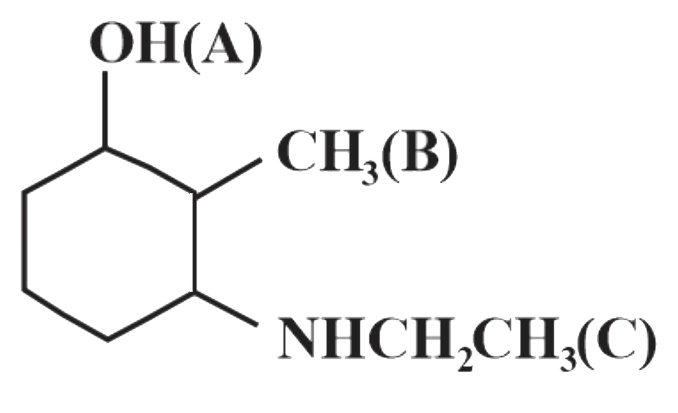
The current carrying ions are not necessarily discharged at the electr

Suggest the most important type of intermolecular attractive interac

General Tests, Processes and Apparatus

WO2017213502A1 - Aqueous composition for livestock animals - Google Patents

At `300K,36g` of glucose present per litre in its solution had an osmotic pressure `4.98 ` bar. If

Electrolytes in the ICU

Solved] At 300 K,36 g of glucose present in a litre of its solution has ..

B14. At 300k, 30g of glucose, C6H1206 present per litse en its solutior has an osmotic pressure of 4.98 bar. If the asmotic pressure of another glucose solution is 1.52 bar the
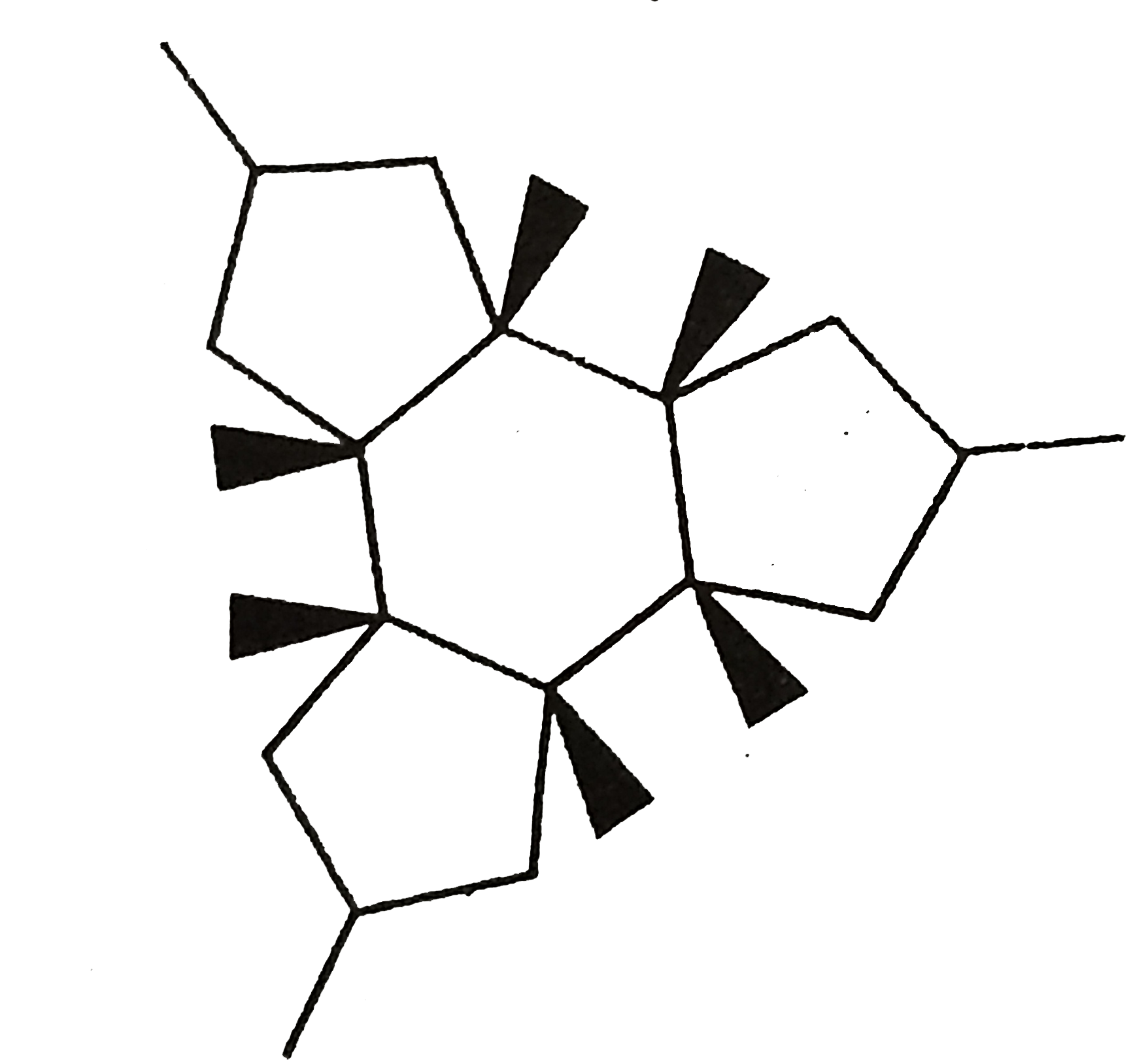
Number of chiral centres in Pencillin is







