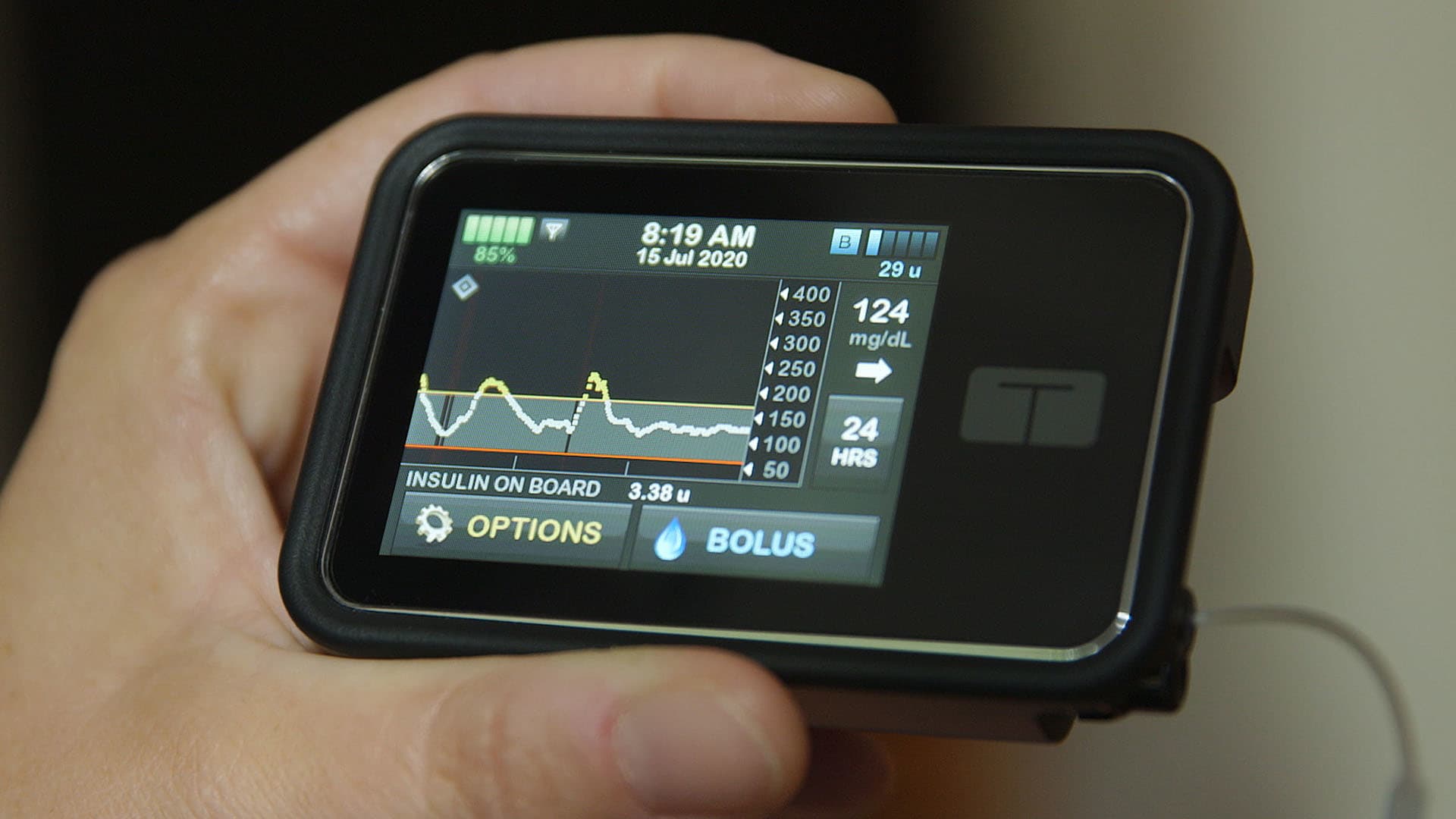
Modular Medical submits next-gen insulin pump for FDA clearance

Modular Medical (Nasdaq:MODD) announced today that it submitted its next-generation MODD1 insulin pump to the FDA for 510(k) clearance.

Upgraded Sapphire Infusion Pump System Receives FDA Clearance - OR Today

Drug Delivery Business News on LinkedIn: Abbott FreeStyle Libre named 'best medical technology' in 50 years

Novocure touts combined TTF, chemotherapy mesothelioma study data - MassDevice
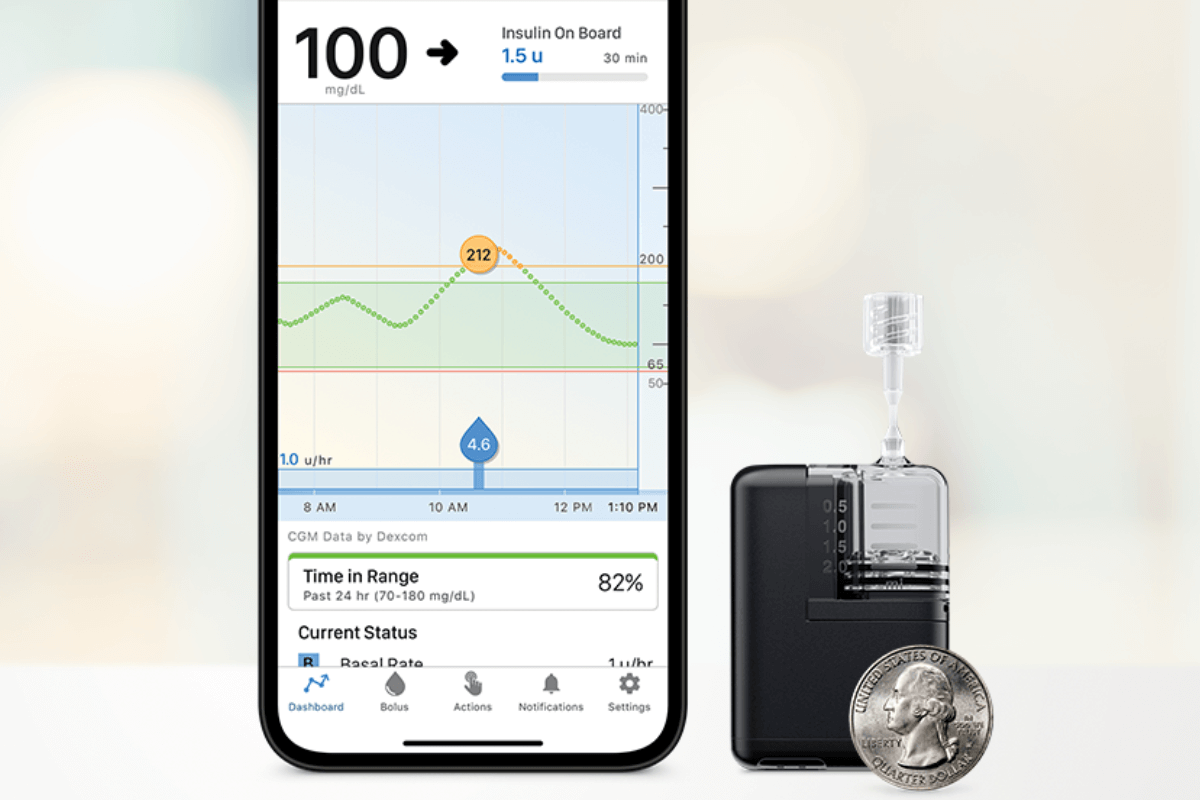
The Smallest Artificial Pancreas System Receives FDA Clearance - JDRF

FDA roundup: The major device, app, and algorithm approvals of 2018 (so far)

A Guide to Insulin Pumps and New Diabetes Technology

FDA clears icometrix' MRI quantification software - MassDevice

Welldoc says CGM, digital health combo offers better glycemic outcomes

ex99_1page003.jpg

Quality Means Business on LinkedIn: Modular Medical submits next-gen insulin pump for FDA clearance

In the News.. weight loss & cancer study for T2D, new pump submitted, Summer Olympic hopeful with T1D and more! - Diabetes Connections

Drug Delivery Business News on LinkedIn: Medicare covers Medtronic MiniMed 780G automated insulin pump

Insulin Patch Pumps Market Size, Growth, Trends And Report, 2024-2033