An ideal gas is taken from (Pi, Vi) to (Pf, Vf) in three different ways. Identify the process in which the work done on the gas the most. - Physics

An ideal gas is taken from (Pi, Vi) to (Pf, Vf) in three different ways. Identify the process in which the work done on the gas the most.

General Physics II - The University of Alabama

Thermodynamic Principal #chemical engineering microproject

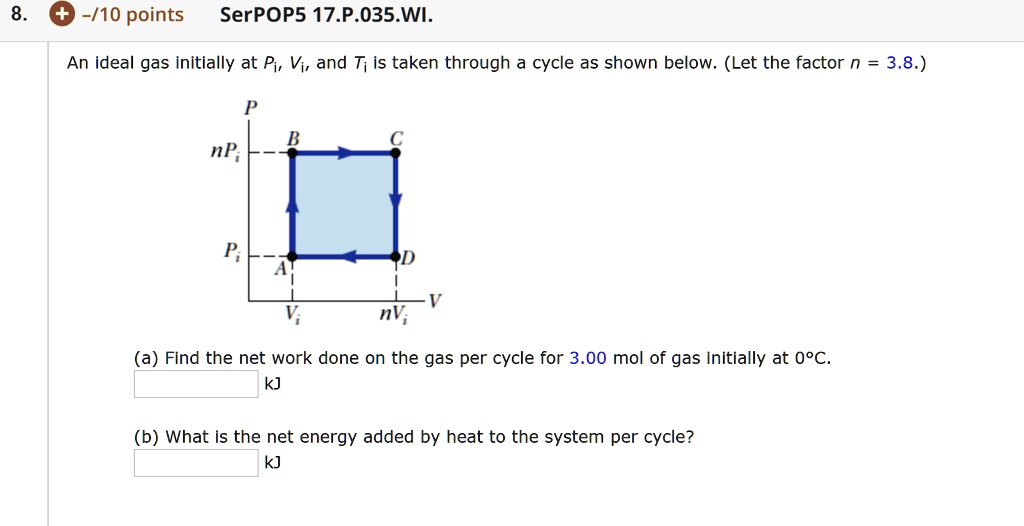
Physics Web Assign Ch 12 #8

P) Thermodynamics, PDF, Gases
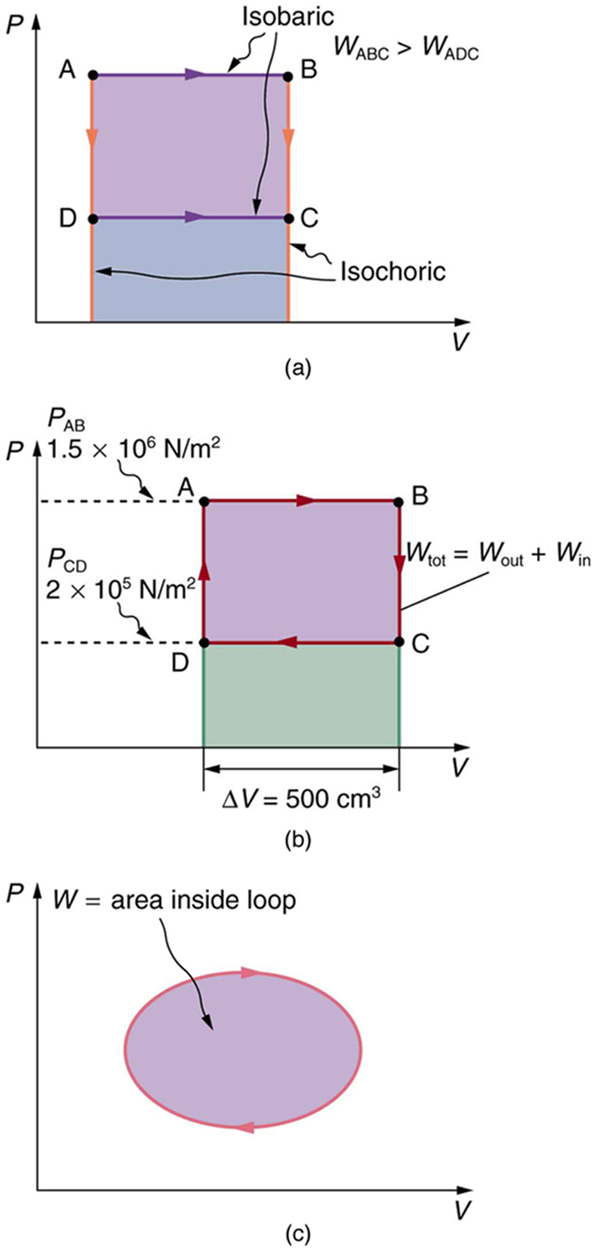
15.2 The First Law of Thermodynamics and Some Simple Processes – College Physics

An ideal gas expands from volume V_1 to V_2. This may be achieved by either of three processes: isobaric, isothermal and adiabatic. Let Delta U be the change in internal energy of
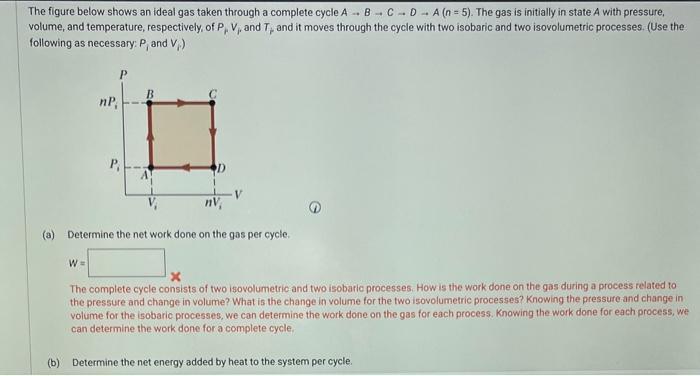
Solved The figure below shows an ideal gas taken through a

An ideal gas is taken from (Pi, Vi) to (Pf, Vf) in three different ways. Identify the process in which the work done on the gas the most. - Physics

Graphically show the total work done in an expansion when the state of an ideal gas is changed reversibly and isothermally from pi, Vi to pf, Vf With the help of p

Heat & Thermodynamics [3 ed.]

The origin of irreversibility and thermalization in thermodynamic processes - ScienceDirect

Solved An ideal gas is taken through two processes in which
IJMS April-1 2021 - Browse Articles

Ideal Gas Law Paper