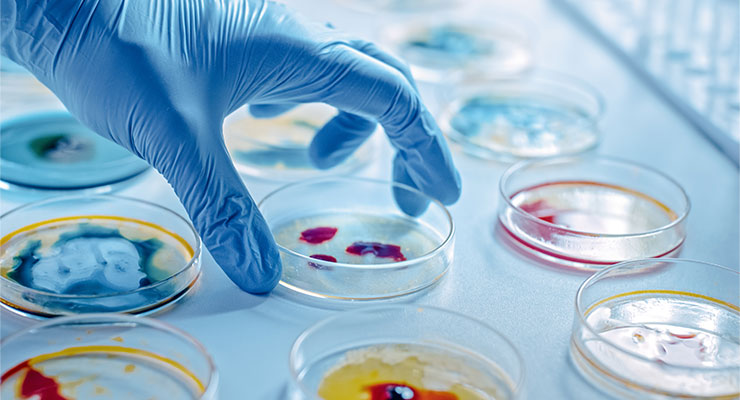
Microbiological Media Management - SOP & Guideline - Pharma Beginners

Standard Operating Procedure (SOP) and Guideline for the Receipt, Storage, Preparation, Growth Promotion Test, use, and Disposal of microbiological media.

Validation of Microbiological Methods
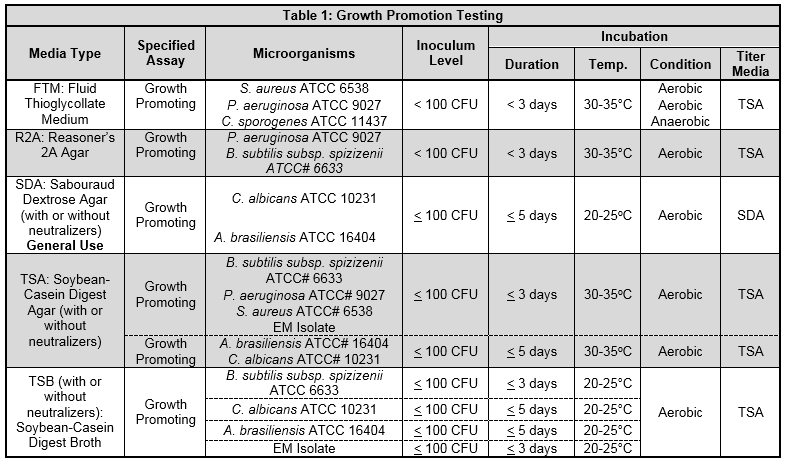
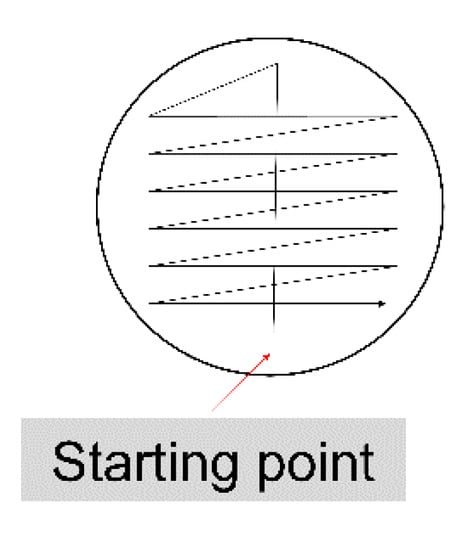
How To Establish Growth Promotion Tests For Pharmaceutical Culture Media
Diagnostics, Free Full-Text

Culture media

BACT/ALERT® Culture Media Bottles
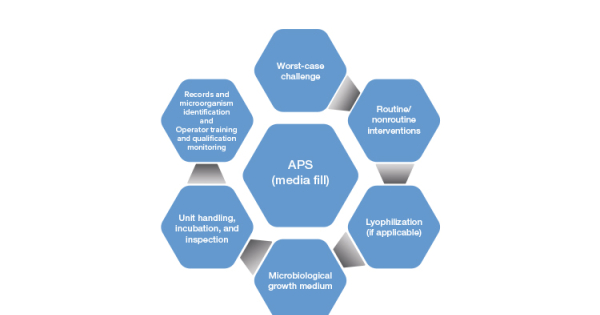
Validation of Aseptic Processes Using Media Fill

Microbiological Analysis of Water & its SOP

MICRO 4 SOP For Microbial Monitoring in Drain Point of Pharmaceutical Manufacturing Sites

Organoids, Free Full-Text

Culture techniques, procedures and regulations that should be included

Method Changes for Bacterial Endotoxins Testing (BET): Steps to
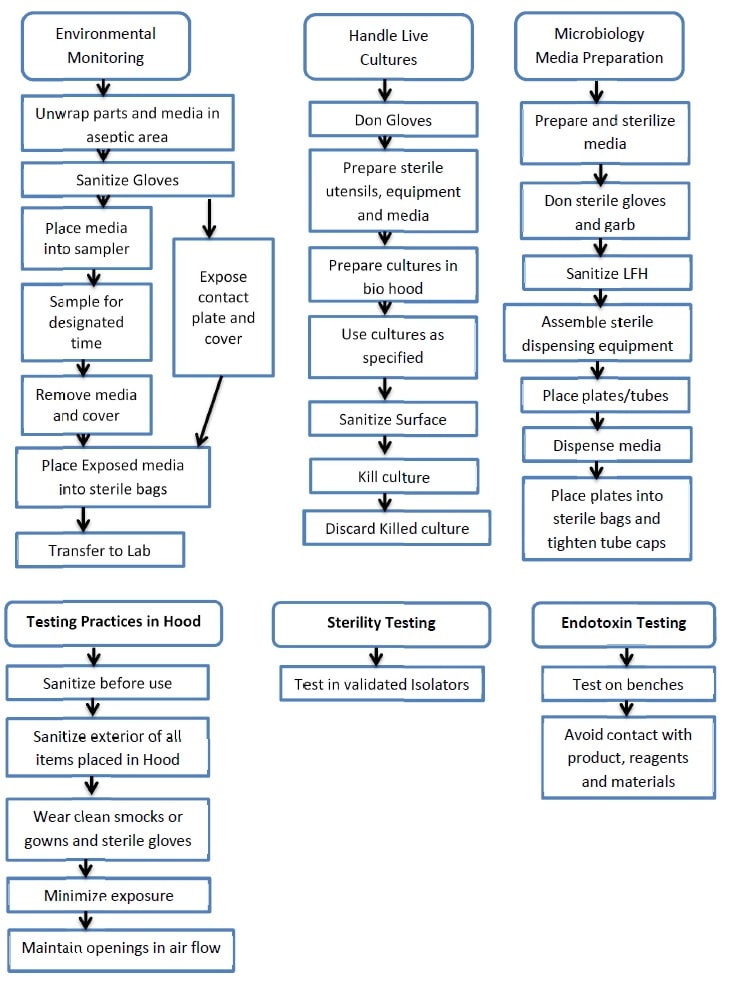
Aseptic Technique for Microbiological Testing - Pharma Beginners
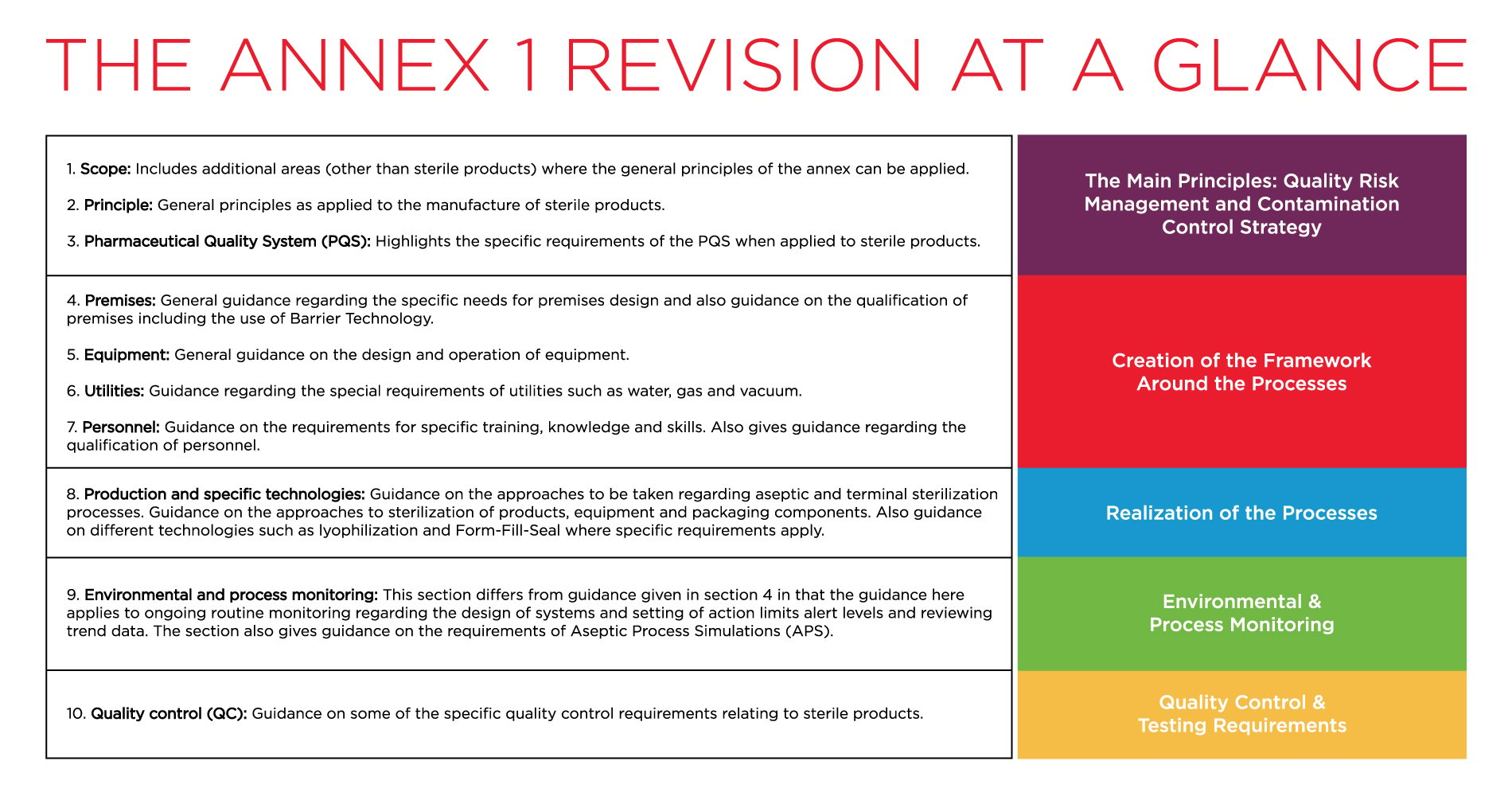
EU GMP Annex 1
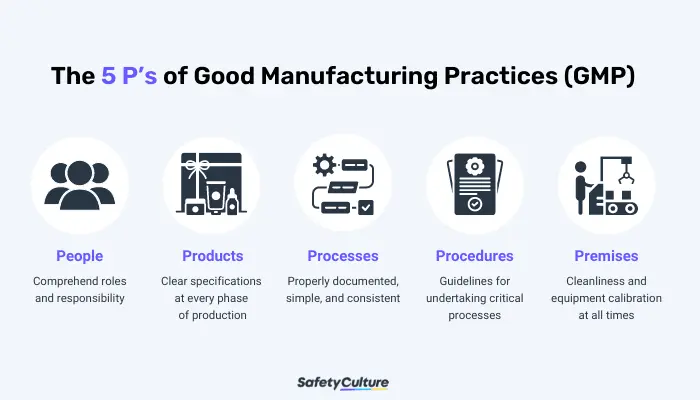
What is GMP, Good Manufacturing Practices