Preparation of Standard Solution of Sodium Carbonate - Chemistry

A common primary standard for standardizing strong acids is sodium carbonate (Na2CO3).For acid-base titration, it is customary to prepare solutions of an acid and base of the desired concentration. Visit BYJU
A common primary standard for standardizing strong acids is sodium carbonate (Na2CO3).For acid-base titration, it is customary to prepare solutions of an acid and base of the desired concentration. Visit BYJU'S to understand more about it.

Solved you are going to prepare a standard solution of

How to Make Sodium Carbonate Solution

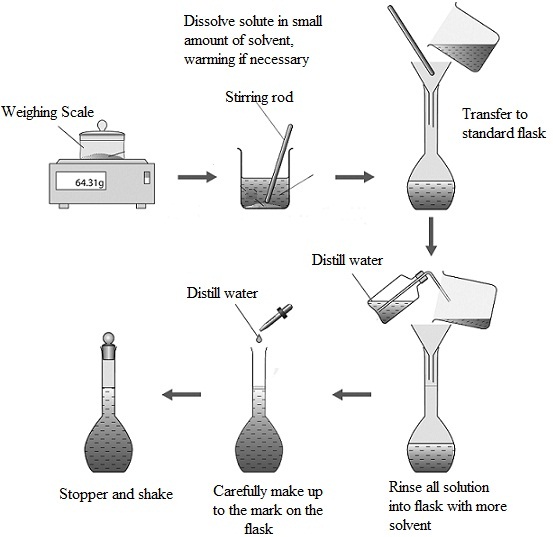
Preparing a standard solution - sodium carbonate

Task 1: Preparation of standard solutions Purpose To

Preparing a standard solution

Solved I need help with part 5, 8 and 9 calculations I dont
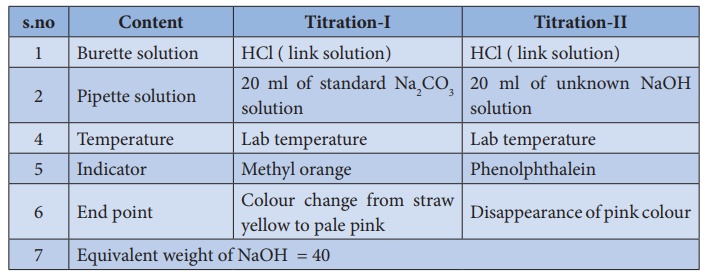
Estimation of sodium hydroxide - Volumetric Analysis

CHM231 - good - CHM 231 : ANALYTICAL OF CHEMISTRY LAB REPORT

Sodium carbonate-bicarbonate eluent solution 3 mM Na₂CO₃/2 mM NaOH
Acids-Bases and Salts-Volumetric analysis, Chemistry tutorial

Volumetric Analysis 1 To make a standard solution of sodium

Experiment 1, PDF, Sodium Carbonate