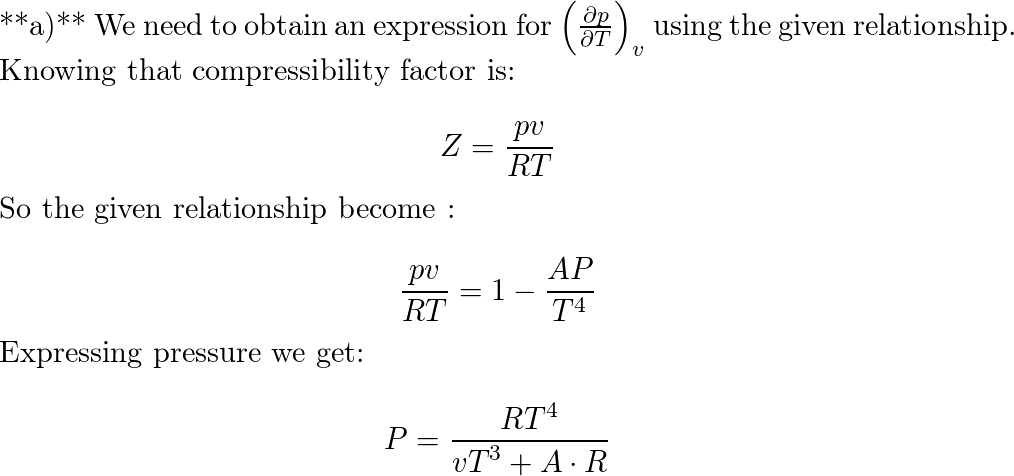Solved RT B 2. The compressiblity factor for a gas is

Answer to Solved RT B 2. The compressiblity factor for a gas is

The compressibility factor of a gas is defined as Z=P V / R T. The compressibility factor of idea
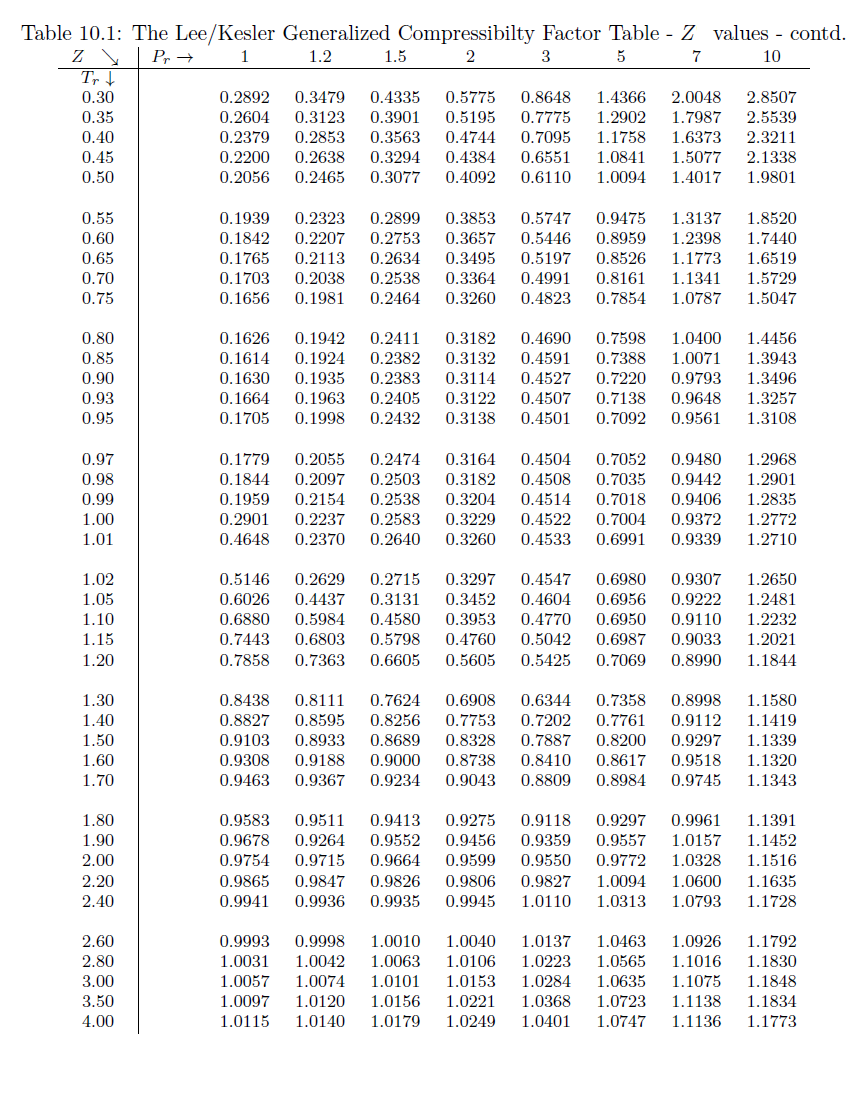
PVT Data from Compressibility Factor Table

Deviations from ideal gas behaviour, intermolecular forces, Van der Waals equation of state, compressibility factors and the critical pressure and critical temperature of a gas revision notes doc brown's chemistry UK advanced

Solved] The compressibility factor for an ideal gas is

Gaseous State

The compressibility factor for a real gas at high pressure is .

Physical Chemistry The Compression Factor (Z) [w/1 example]

Which of the following statements is/are correct? (a) all real gases are less compressible

Procedure calculates base gas compressibility factors

Gas compressibility factor Z: Ideal gas vs Real gas

for a real gas at 25∘C temperature and high pressure (99 bar) the value o..
The given graph represent the variation of z compressibility

Compressibility Factor Z Important Concepts and Tips for JEE Main
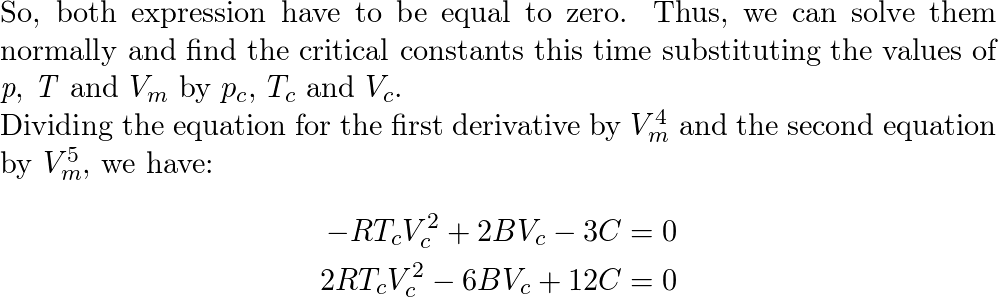
A scientist proposed the following equation of state $p= ra

Deviation of Real Gases from Ideal Gas Behaviour - GeeksforGeeks