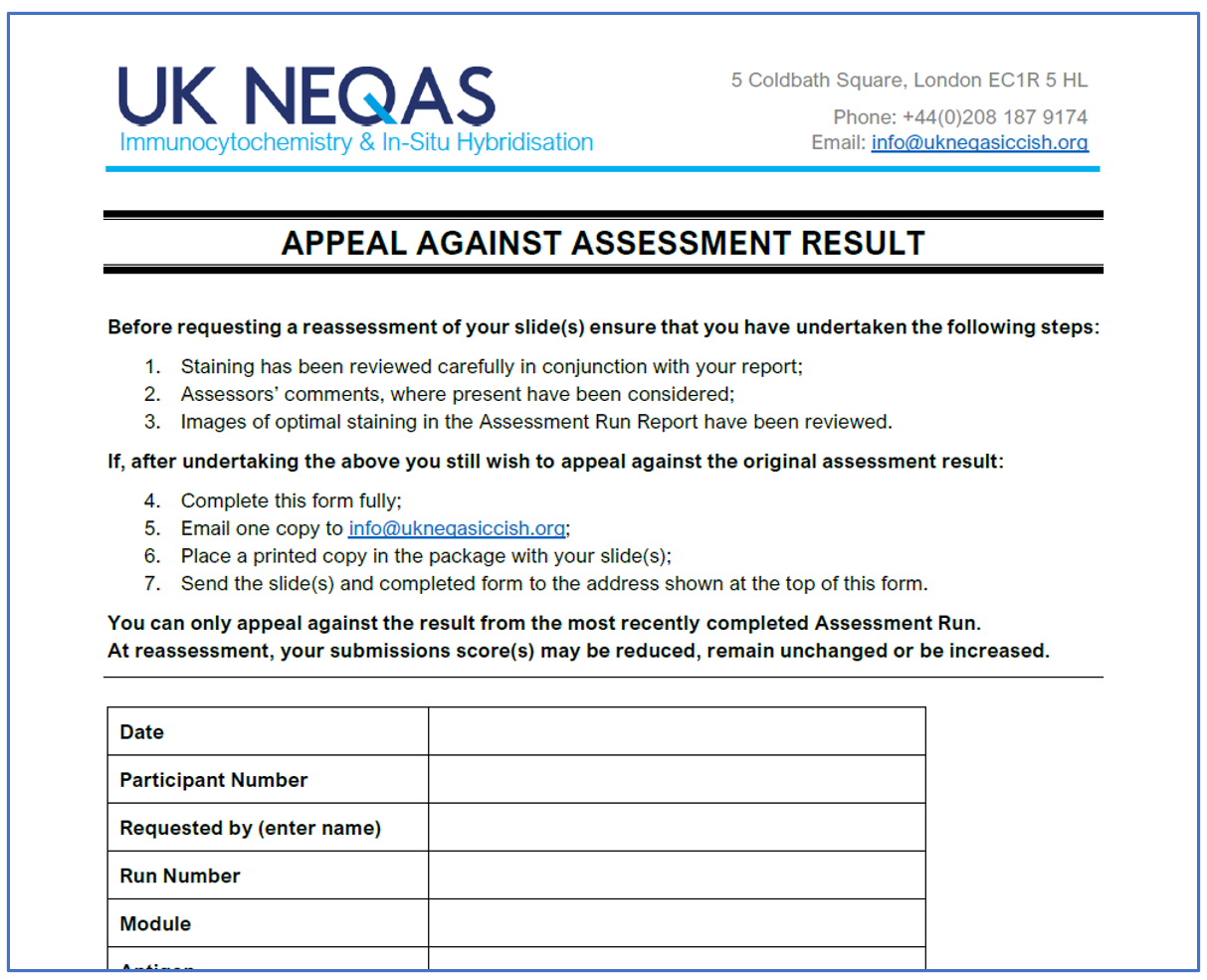
Useful Forms – UK NEQAS – ICC & ISH

PDF) Breast cancer biomarkers in clinical testing: analysis of a UK NEQAS ICC & ISH database containing results from 199,300 patients: Clinical testing for breast cancer biomarkers

Assessment Procedure – UK NEQAS – ICC & ISH

United Kingdom National External Quality Assessment Service for

chantell hodgson (@ShaggydogHodg) / X
e-Journal – UK NEQAS – ICC & ISH
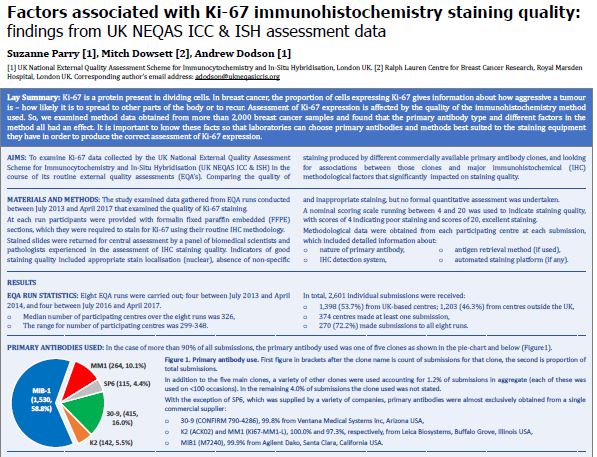
Publications – UK NEQAS – ICC & ISH

Publications – UK NEQAS – ICC & ISH

Biomarker testing in oncology – Requirements for organizing external quality assessment programs to improve the performance of laboratory testing: revision of an expert opinion paper on behalf of IQNPath ABSL

Concordance of HER2-low scoring in breast carcinoma among expert pathologists in the United Kingdom and the republic of Ireland –on behalf of the UK national coordinating committee for breast pathology - The
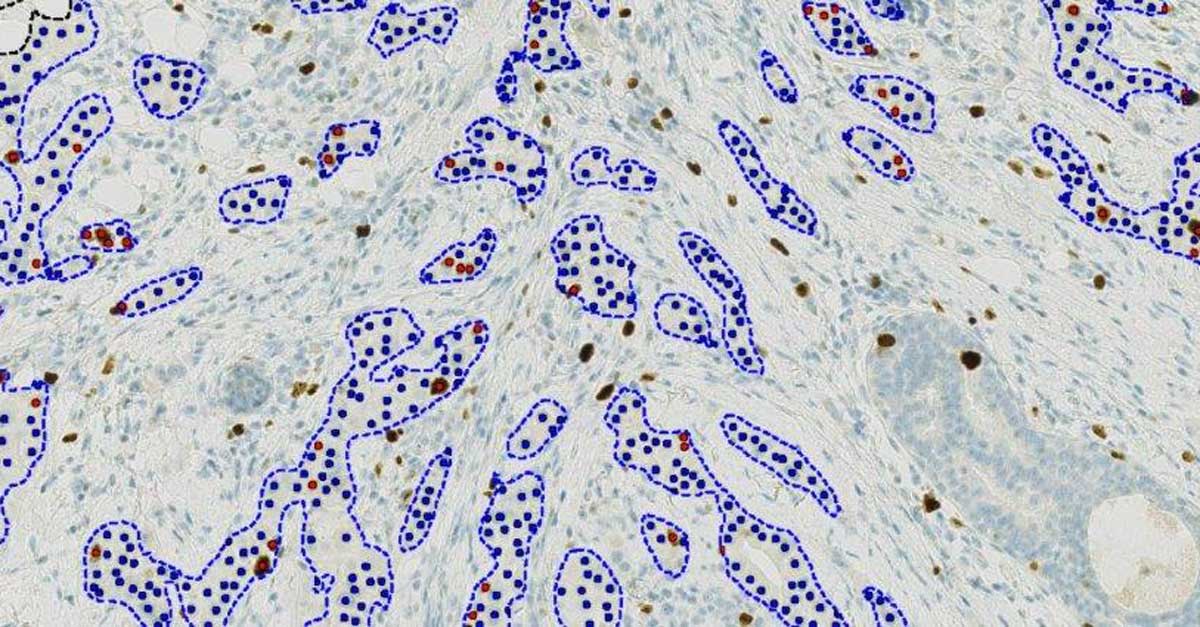
Visiopharm launches IVDR-cleared and fully automated next-generation Ki67 algorithm for IHC biomarker analysis - Visiopharm

Useful Forms – UK NEQAS – ICC & ISH

PDF) Biomarker testing in oncology – Requirements for organizing external quality assessment programs to improve the performance of laboratory testing: revision of an expert opinion paper on behalf of IQNPath ABSL

Balancing Performance and Sustainability in Clinical Labs

Useful Forms – UK NEQAS – ICC & ISH
Participant Area - UK NEQAS for H&I