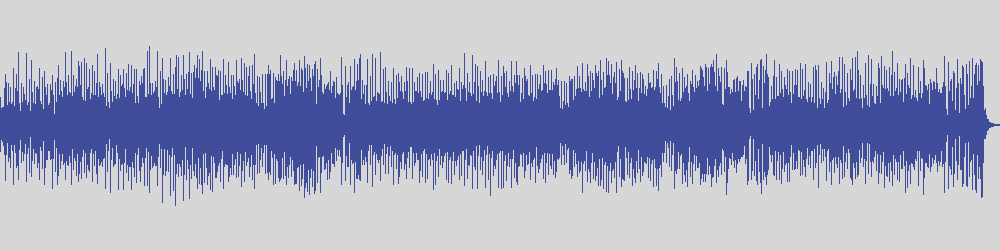
Solved Write the conjugate base for the species shown below.
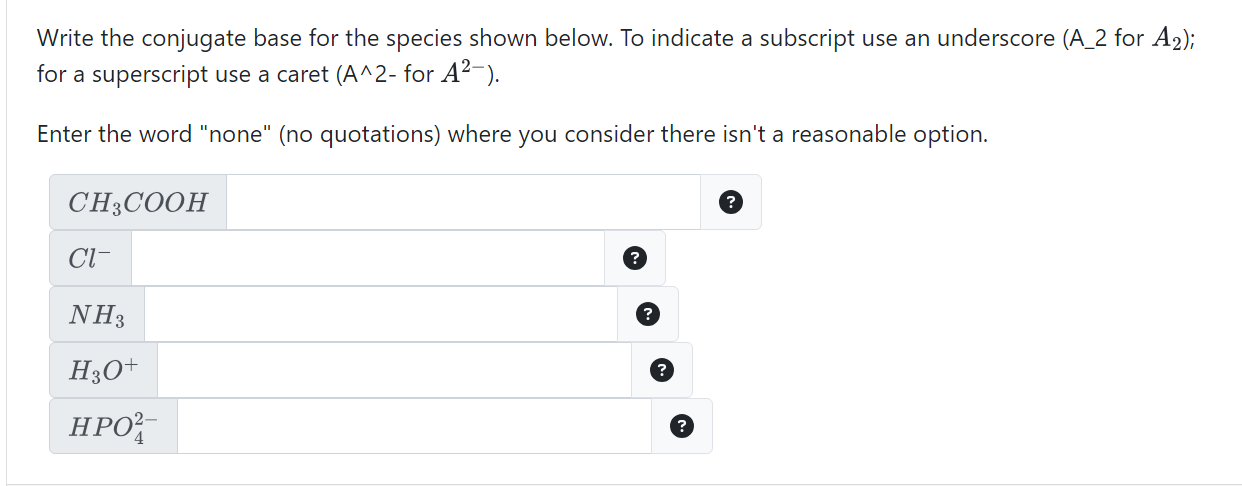
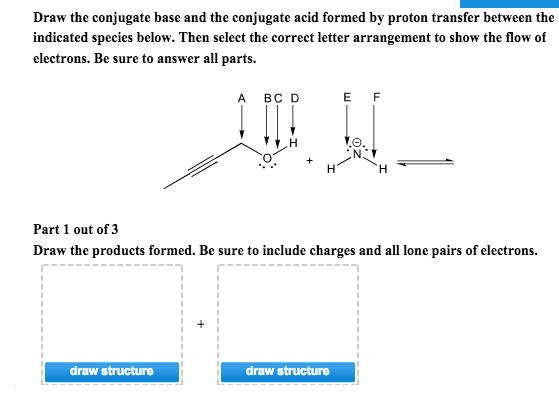
Solved Draw the conjugate base and the conjugate acid formed

Write the formula for the conjugate base of each acid. a. HCl

The species: displaystyle { H }_{ 2 }O,;{ HCO }_{ 3 }^{ - },;{ HSO }_{ 4 }^{ - } and { NH }_{ 3 } can act both as Bronsted acids and bases. For each give the corresponding conjugate acid and base.

Acid Base Reactions In Organic Chemistry – Master Organic Chemistry
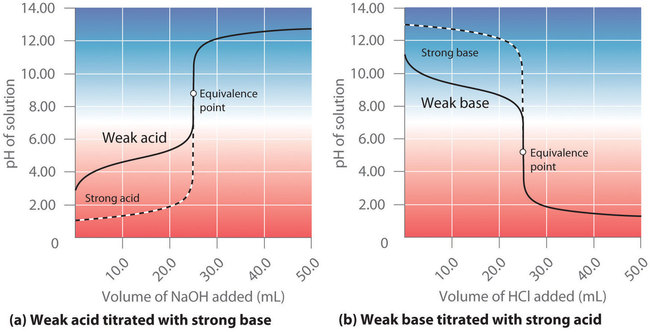
15.6: Acid-Base Titration Curves - Chemistry LibreTexts
2.6: Acids and Bases - The Brønsted-Lowry Definition - Chemistry LibreTexts
Solved] organic chemistry la. For the following reaction, label the
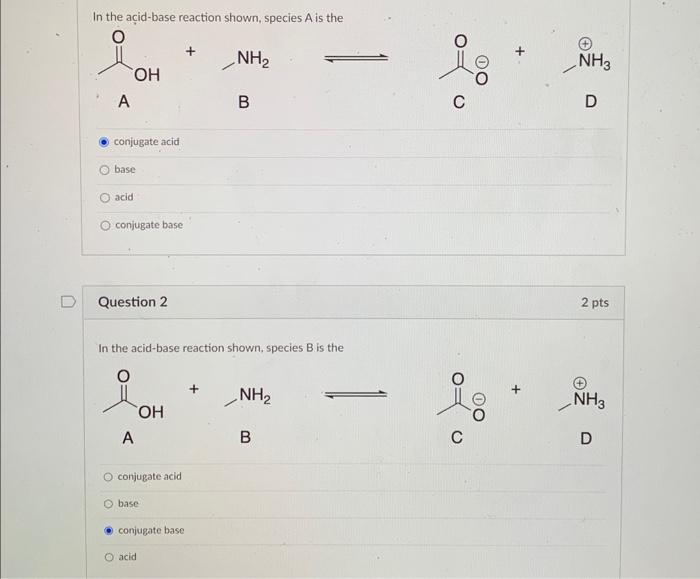
Solved In the acid-base reaction shown, species A is the A B

The following aqueous species constitute two conjugate acid
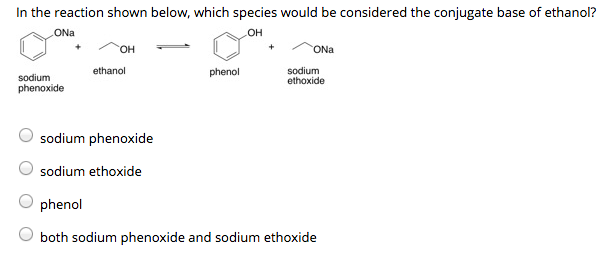
Solved In the reaction shown below, which species would be
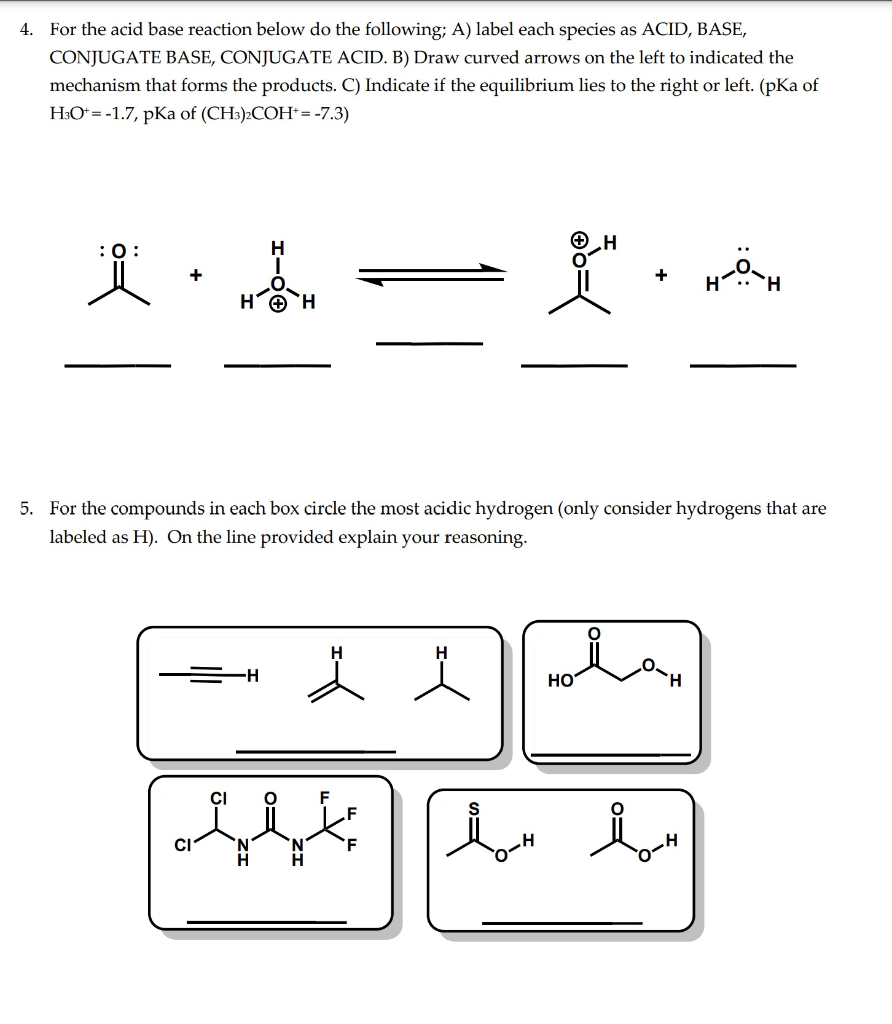
Solved 4. For the acid base reaction below do the following;