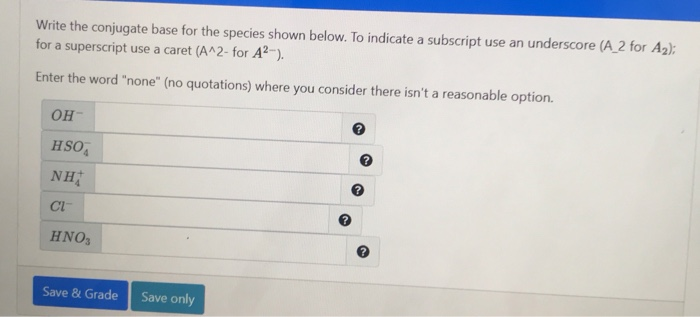
Solved Write the conjugate base for the species shown below
Description
/chapter3/pages33and34/page33and34_files/aqh3o.png)
Chapter 3
Solved] Please help me answer questions below Write the formula of
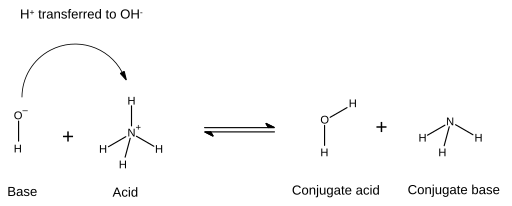
Conjugate (acid-base theory) - Wikipedia
Sn2

For the chemical equations shown below, label each reactant as
What is ammonium or ammonium ion?

The following aqueous species constitute two conjugate acid

SOLVED: Write the conjugate base for the species shown below. To
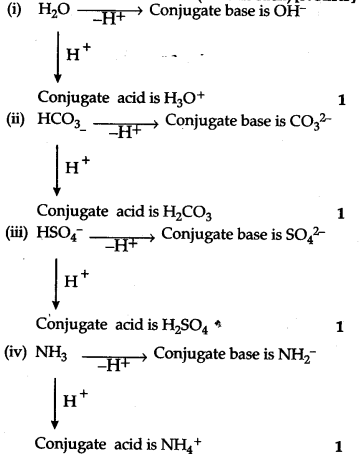
The species${{H}_{2}}$O, H${{CO}_{3}}$, HS${{O}_{4}}$ and N${{H}_{

The species: displaystyle { H }_{ 2 }O,;{ HCO }_{ 3 }^{ - },;{ HSO
Advanced BioMatrix - PureCol® EZ Gel, Solution, 5 mg/ml (bovine) #5074
Related products
You may also like
$ 29.50USD
Score 4.5(389)
In stock
Continue to book
You may also like
$ 29.50USD
Score 4.5(389)
In stock
Continue to book
©2018-2024, paramtechnoedge.com, Inc. or its affiliates






