Solved The compression factor (Z) for a real gas can be


Compressibility Factor of Gas Overview, Equation & Chart

Real gas z-Factor chart [2] Download Scientific Diagram

Explain how the compression factor varies with pressure and

Which of the following statements is/are correct? (a) all real

Compressibility factor, Z of a gas is given as Z=(pV)/(nRT) (i) What

Physical Chemistry The Compression Factor (Z) [w/1 example

physical chemistry - Why do some gases have lower value of Z for a

What is the compressibility factor? What is its value an ideal gas

Gas Compressibility - an overview
What is the value of compressibility factor in terms of vander

the equation of state of a gas is p(v-nb)=rt where b and r are

The compressibility factor of a gas is defined as Z=PV/nRT. The
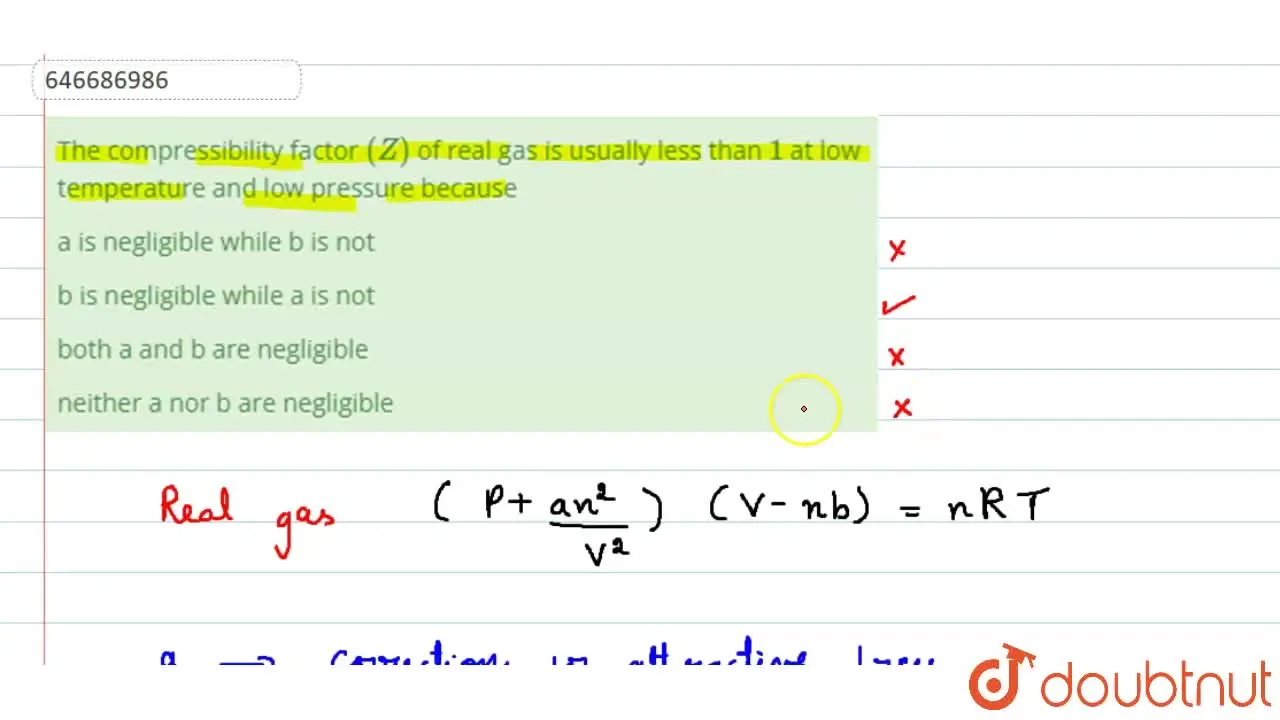
The compressibility factor (Z) of real gas is usually less than 1 at l

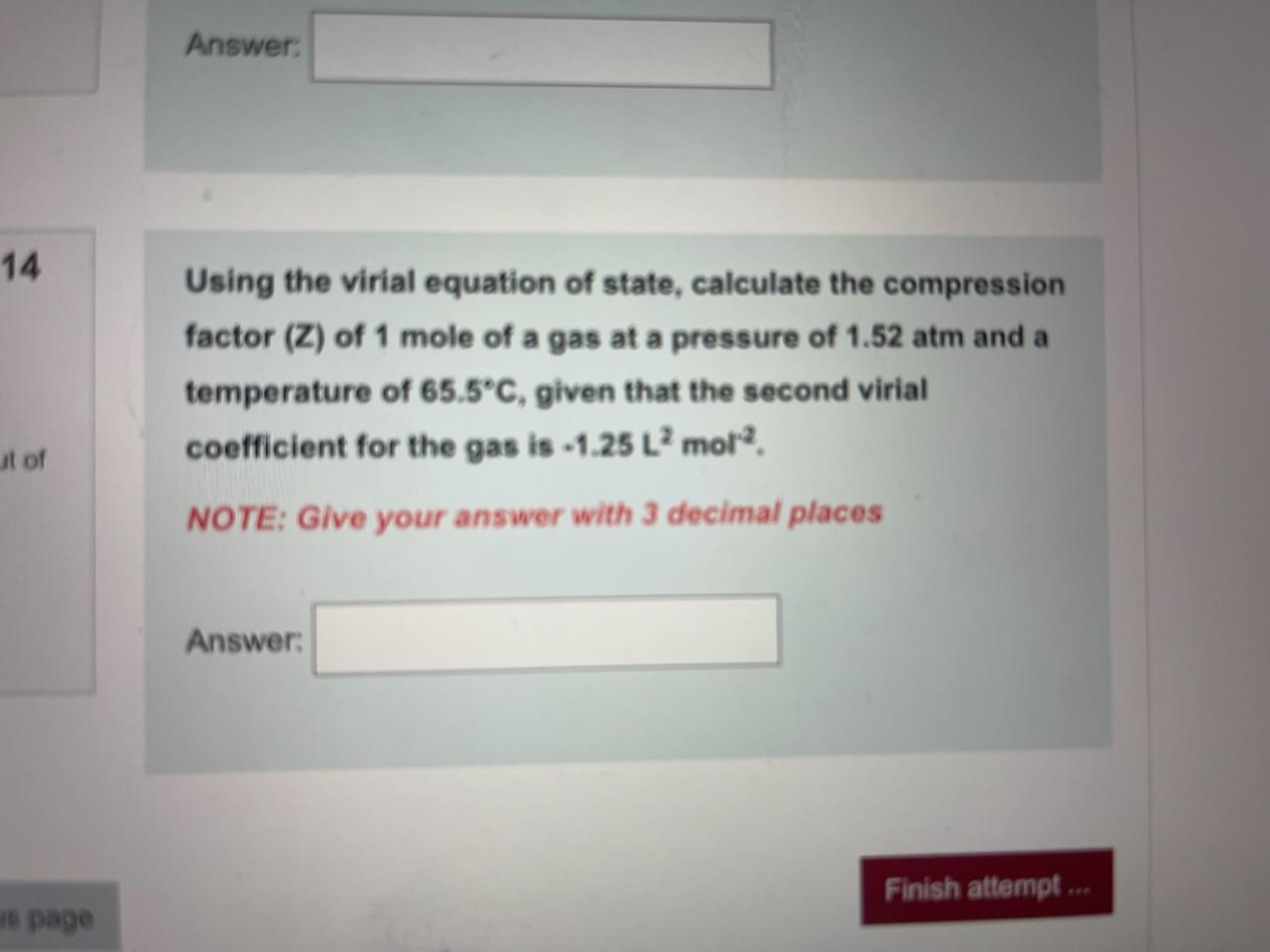
3.3: Real gas and compressibility factor - Engineering LibreTexts