Compressibility factor (Z) for a van der Waals real gas at

Share your videos with friends, family and the world

Non-ideal behavior of gases (article)

At a high pressure, the compressibility factor (Z) of a real gas is us

Punjabi] For a real gas (mol.mass =60) if density at critical point i

Non-Ideal Gas Behavior Chemistry: Atoms First

physical chemistry - Does the van der Waals equation remain valid when repulsive intermolecular forces dominate? - Chemistry Stack Exchange
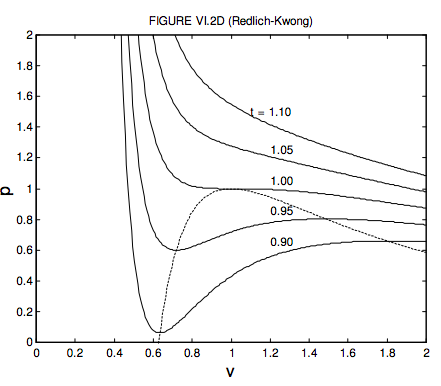
6.3: Van der Waals and Other Gases - Physics LibreTexts

Explain how the compression factor varies with pressure and

6.3: Van der Waals and Other Gases - Physics LibreTexts

Compressibility factor (gases) - Citizendium

Ideal Gas Equation - an overview
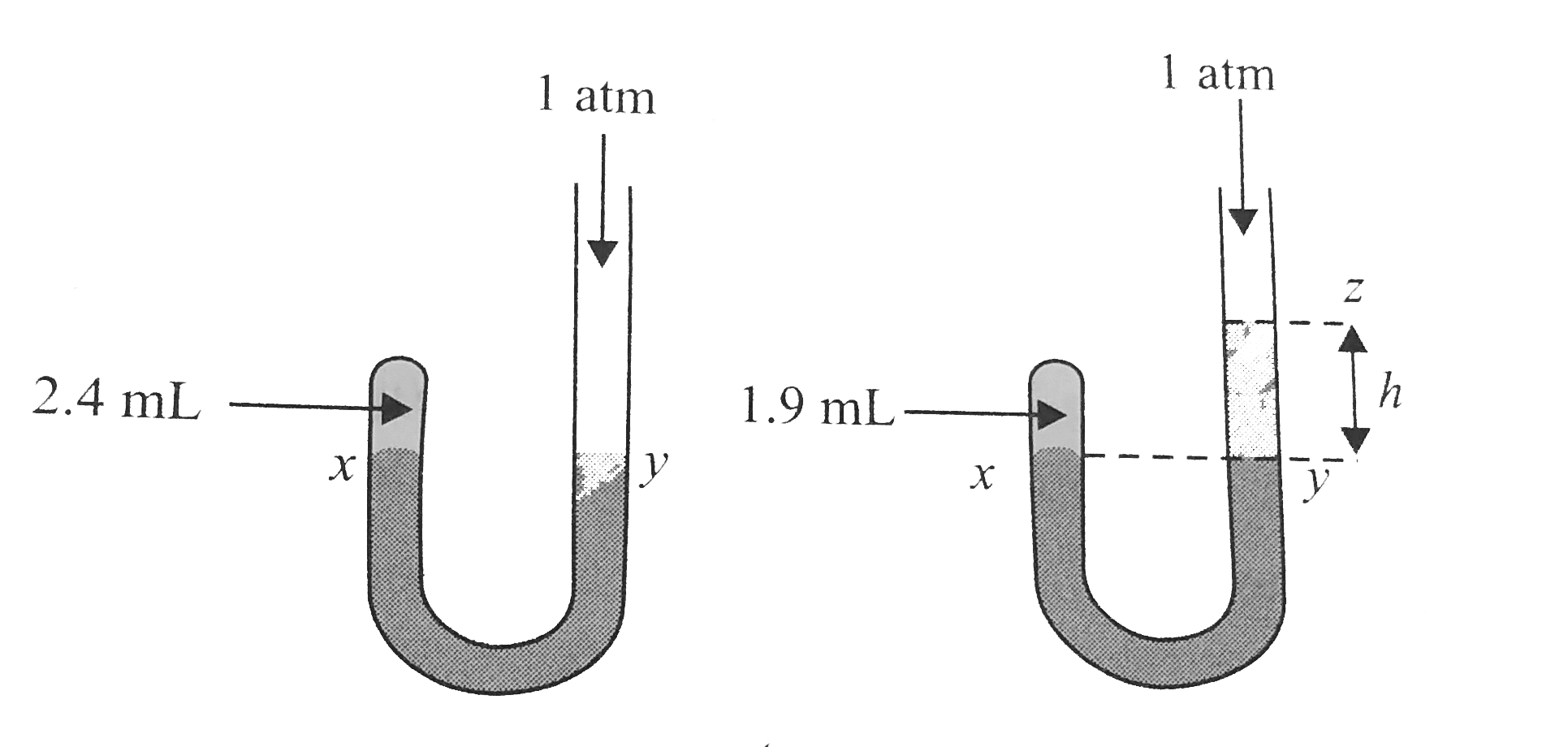
Complete Solutions to Mock Test 1 of chapter MOCK TEST of Class 11 book with complete answers and questions

Compressibility Factor Calculator - File Exchange - MATLAB Central









