What is the change in internal energy (in J) of a system that absorbs 0.464 kJ of heat from its surroundings and has 0.630 kcal of work done on it?

Description
I found an increase of 3100J Have a look

Heat Transfer by J P Holmann

The elastic properties, elastic models and elastic perspectives of
Calculate the change in internal energy of a system if the energy

PDF) Useful conversion factors

Handbook On Energy Conscious Buildings, PDF, Humidity
CaptionSync Smart Player™

Section 4

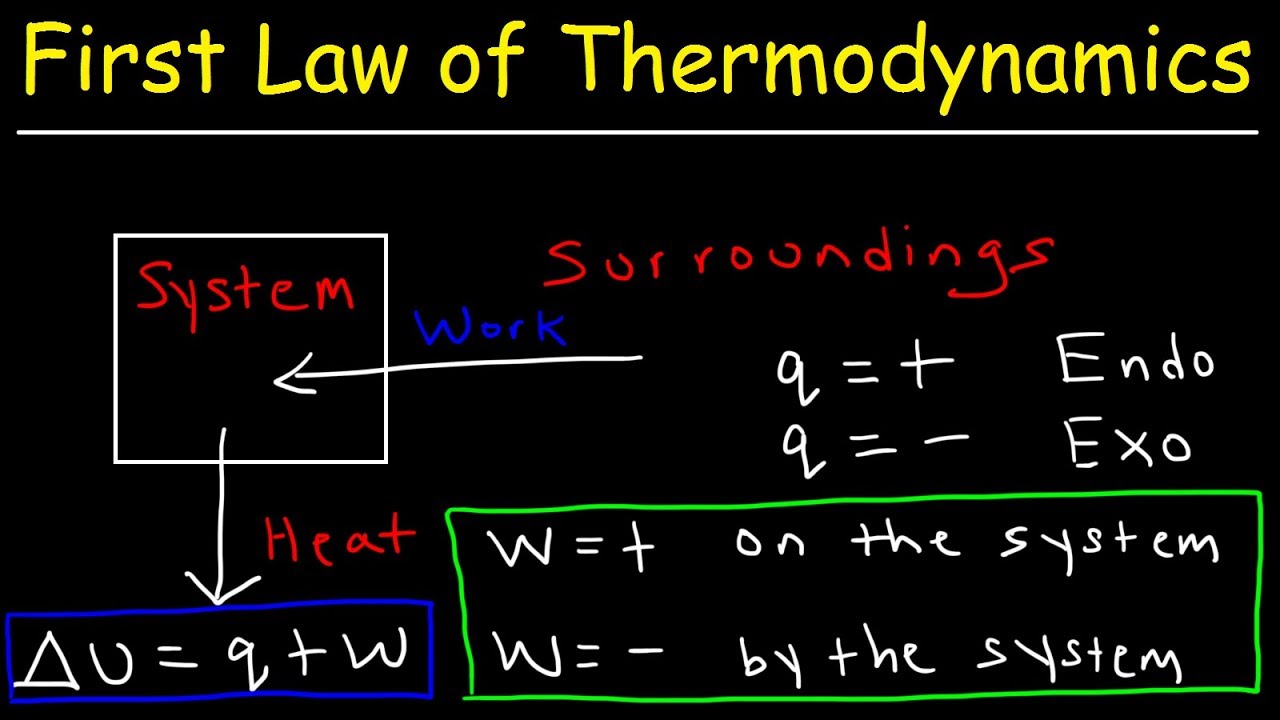
The internal energy of a system changes because the system g
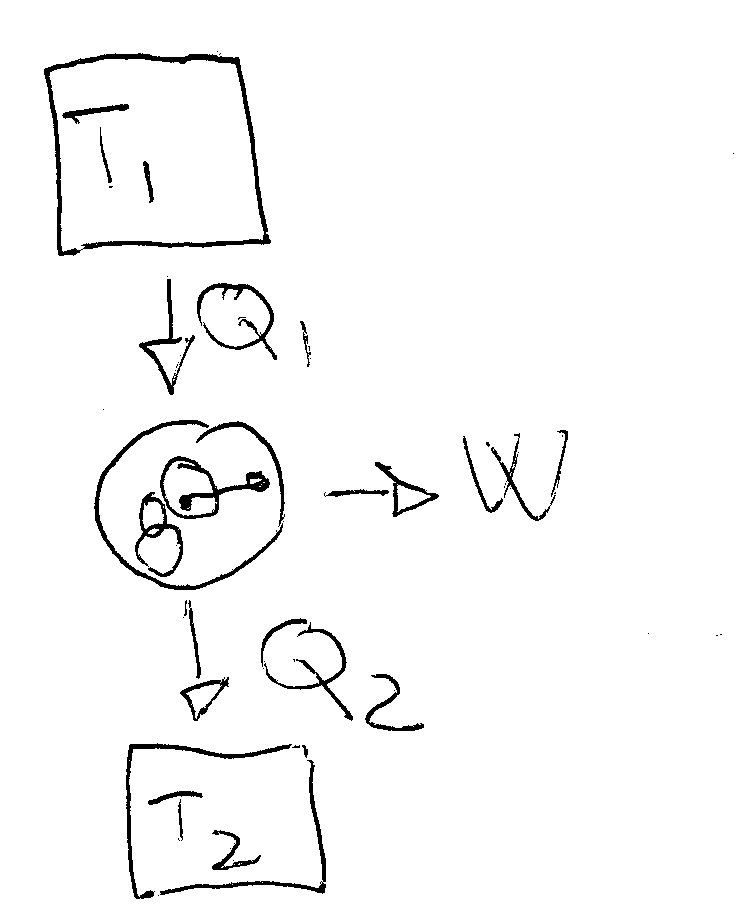
Heat and Work - Physics

Section 4
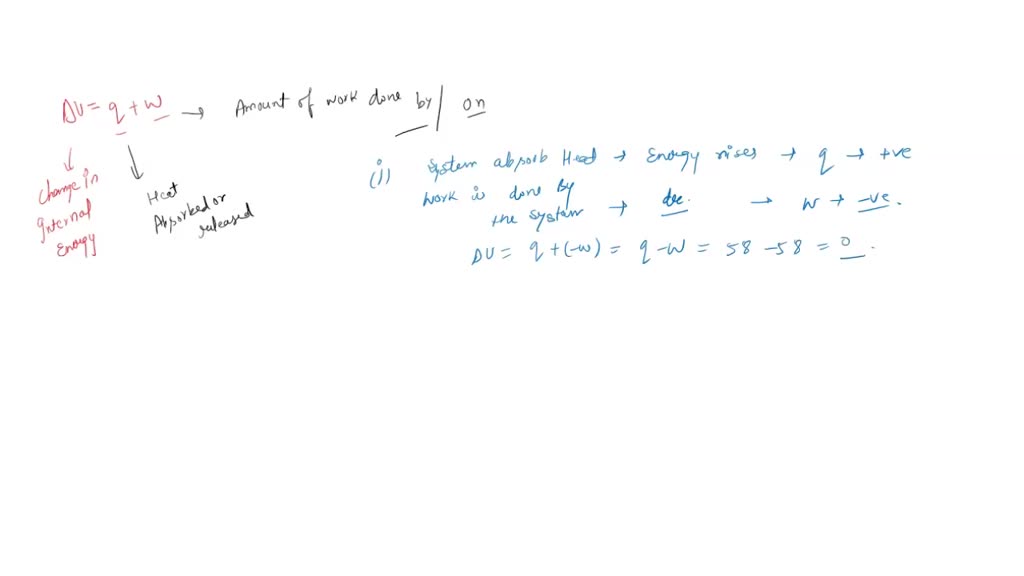
SOLVED: What is the change in internal energy of a system if the
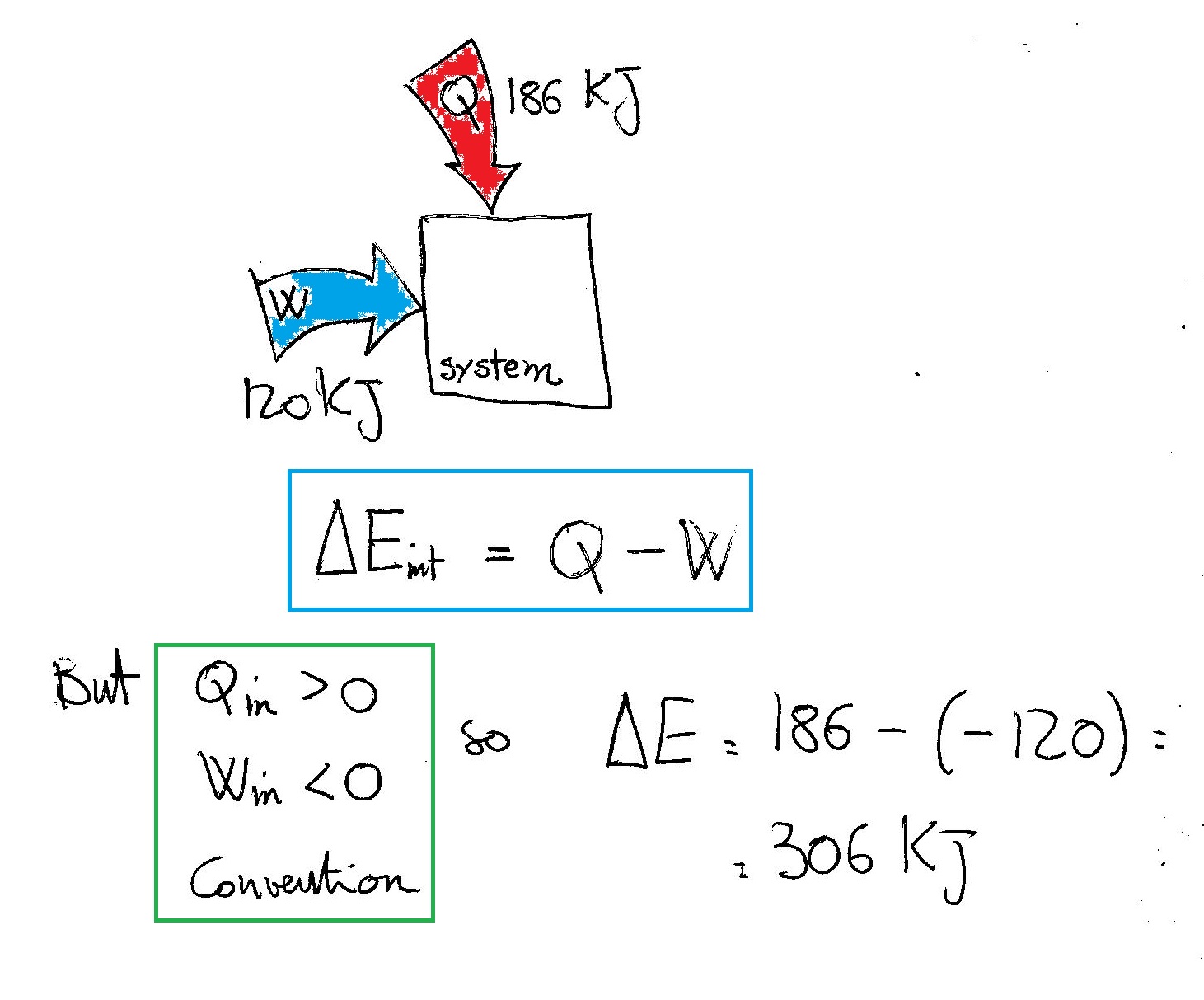
A system absorbs 186 kJ of heat and the surroundings do 120 kJ of

PDF) Shock tube study of normal heptane first-stage ignition near

Irrigation and Drainage Engineering 9783319056999, 3319056999
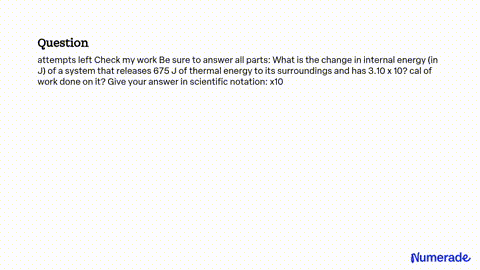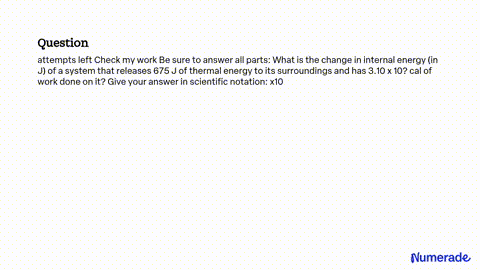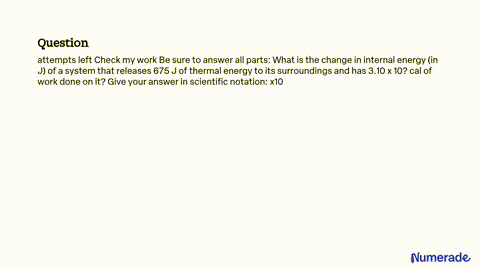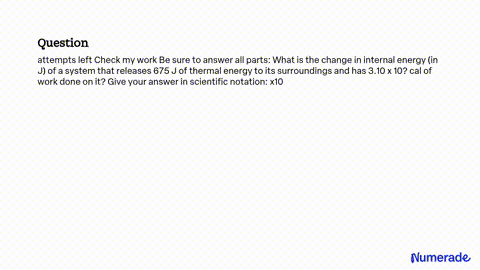
SOLVED: attempts left Check my work Be sure to answer all parts
Related products
You may also like

Spencer Women's Lace Sexy Lingerie Nightwear Two Piece Babydoll Bra Panty Underwear Set Sleepwear XL,Purple

Women's Front Zipper Sports Bras Nylon & Spandex Plus Size Underwear Push up for Running Yoga Sport, Gray 4XL

Dkny Sport Leggings Costco Travel International Society of Precision Agriculture

Dress Red Valentino - Comprar em Desapego Glamour
$ 23.50USD
Score 4.8(664)
In stock
Continue to book
You may also like

Spencer Women's Lace Sexy Lingerie Nightwear Two Piece Babydoll Bra Panty Underwear Set Sleepwear XL,Purple

Women's Front Zipper Sports Bras Nylon & Spandex Plus Size Underwear Push up for Running Yoga Sport, Gray 4XL

Dkny Sport Leggings Costco Travel International Society of Precision Agriculture

Dress Red Valentino - Comprar em Desapego Glamour
$ 23.50USD
Score 4.8(664)
In stock
Continue to book
©2018-2024, paramtechnoedge.com, Inc. or its affiliates