OneClass: For a real gas, the compressibility factor, Z, is

Description

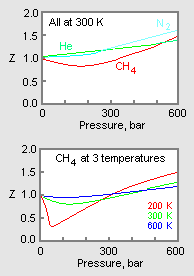
The compressibility factor `(Z)` of real gas is usually less than `1` at low temperature
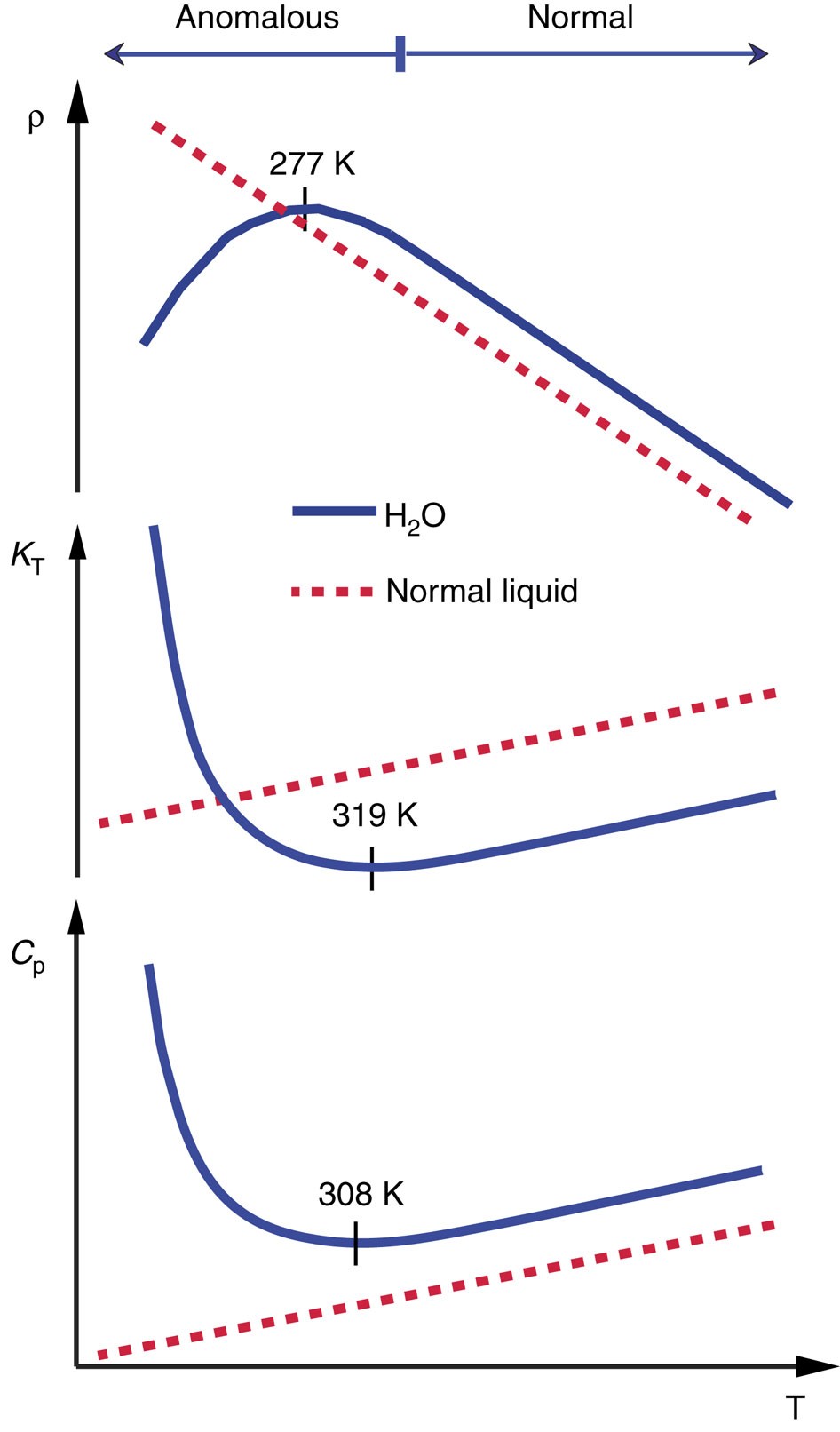
The structural origin of anomalous properties of liquid water

Compressibility Factor of Gas, Overview, Equation & Chart - Lesson

Compressibility factor (gases) - Citizendium
If z<1, does it mean that the gases behave more like perfect or real gases? - Quora

Solved RT B 2. The compressiblity factor for a gas is

OneClass: For a real gas, the compressibility factor, Z, is defined as Z (T, P) = PV/nRT For an ideal

OneClass: The higher the pressure of the gas the lower its compressibility! 2. Find K, for a. a van d

COMPRESSIBILITY factor Z, Using P and v in 3 Minutes!
Related products
$ 11.50USD
Score 4.9(415)
In stock
Continue to book
$ 11.50USD
Score 4.9(415)
In stock
Continue to book
©2018-2024, paramtechnoedge.com, Inc. or its affiliates