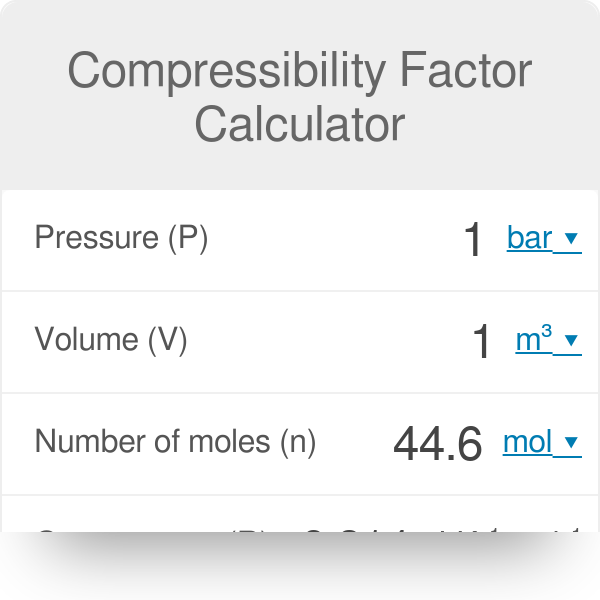
At a high pressure, the compressibility factor (Z) of a real gas is us

Description
At high P. P gt gt (n^(2)a)/(V^(2)) So ‘a’ can be neglected.

Compressibility factor Z - Gaseous State
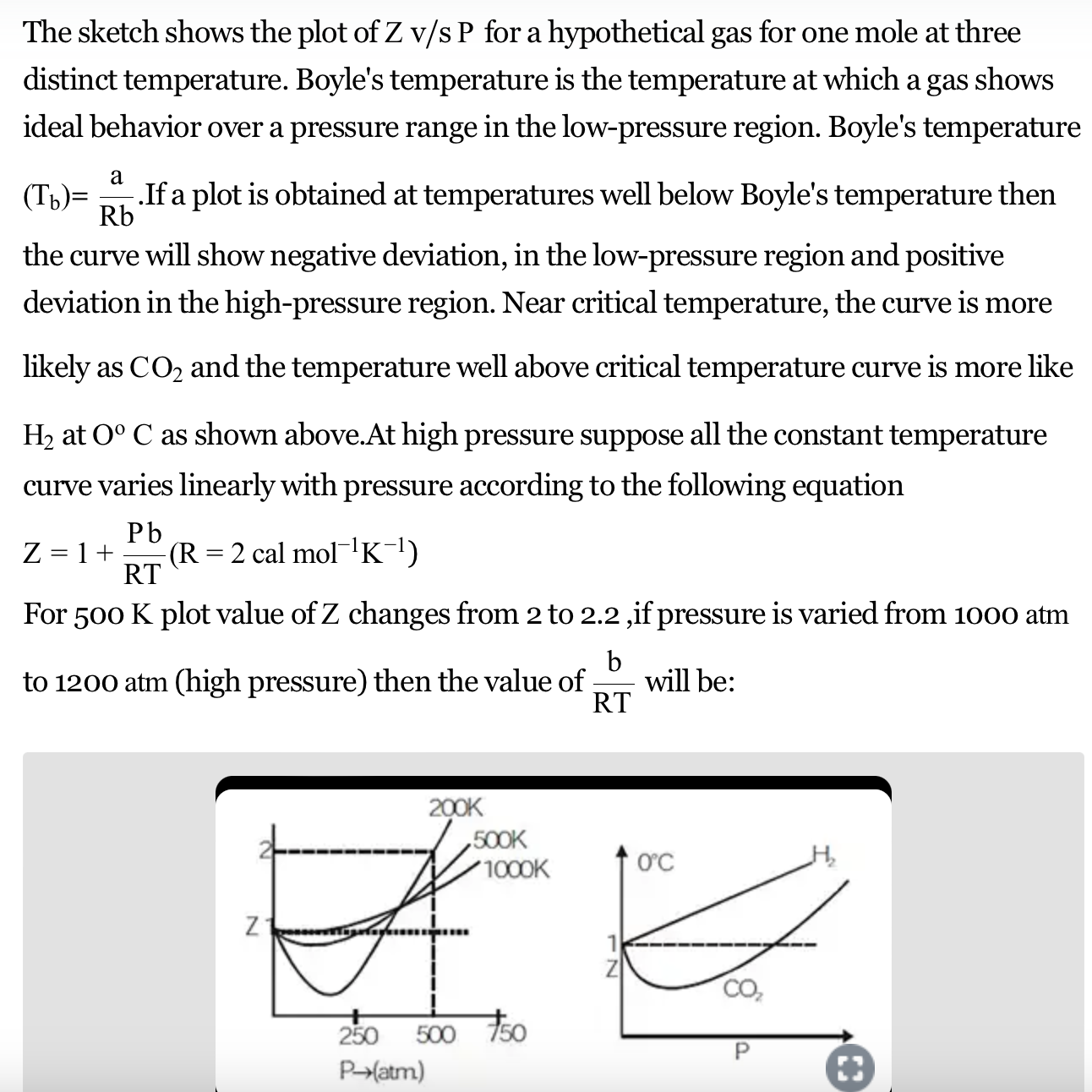
Solved The graph of compressibility factor (Z)v/sP for 1 mol

Solved The graph of compressibility factor (Z)v/sP for 1 mol

Objectives_template

The compressibility factor a real gas high pressure is:-1 - frac{Pb} {RT}1 + frac {RT} {Pb}11 + frac {Pb} {RT}

PDF] Compressibility Chart for Hydrogen and Inert Gases
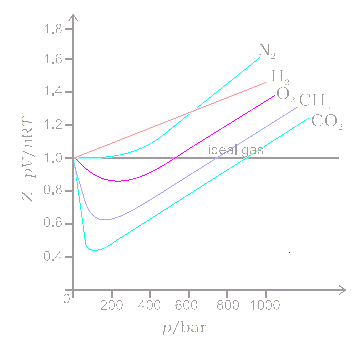
Van der waals equation: Derivation, Explanation

At a high pressure, the compressibility factor (Z) of a real gas is us

Chemistry Desk: Effect of Pressure
Real vs. Ideal Gases — Comparison & Importance - Expii
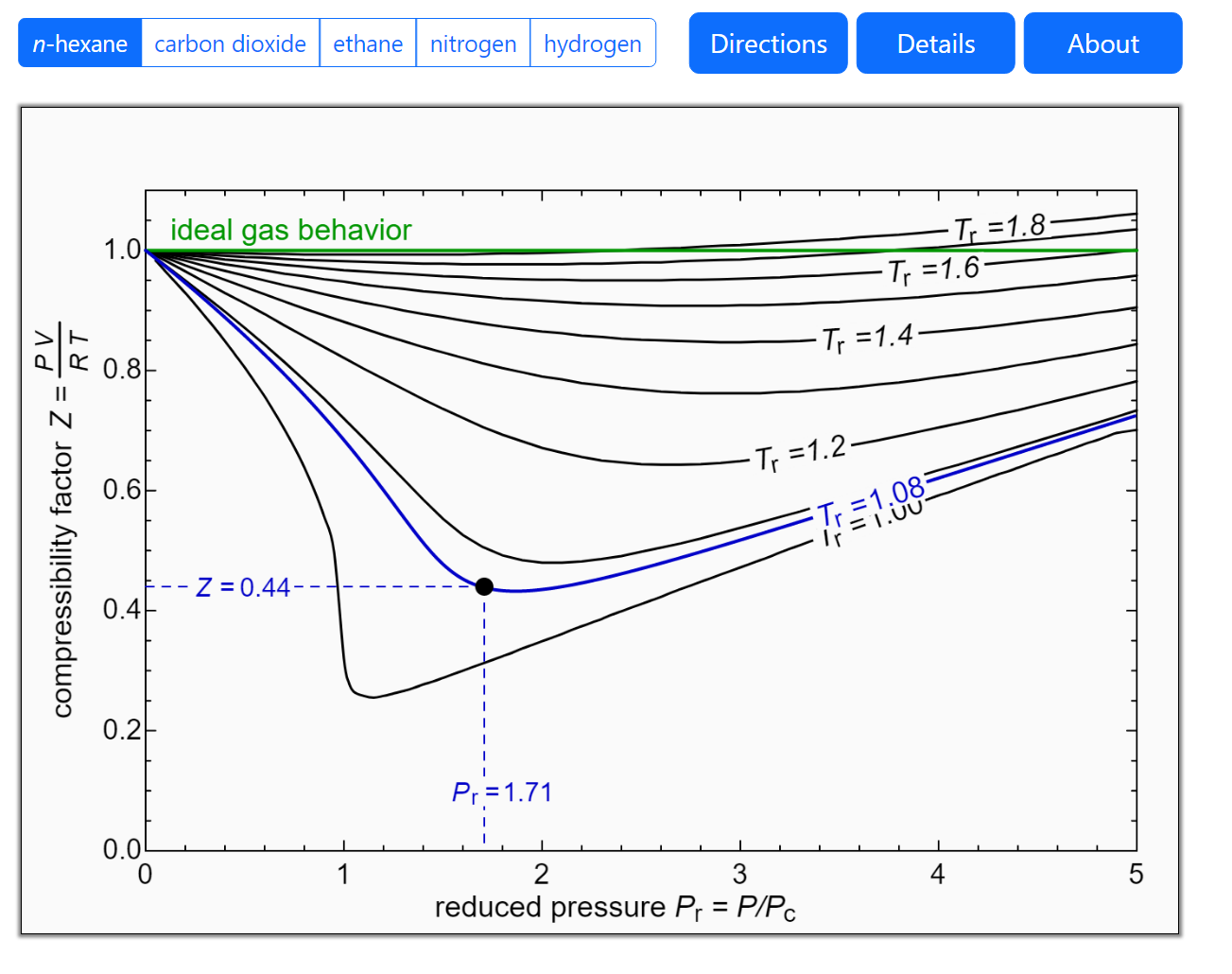
/wp-content/uploads/2023/05/compress
Related products
$ 13.99USD
Score 5(613)
In stock
Continue to book
$ 13.99USD
Score 5(613)
In stock
Continue to book
©2018-2024, paramtechnoedge.com, Inc. or its affiliates