Low coverage whole genome sequencing enables accurate assessment
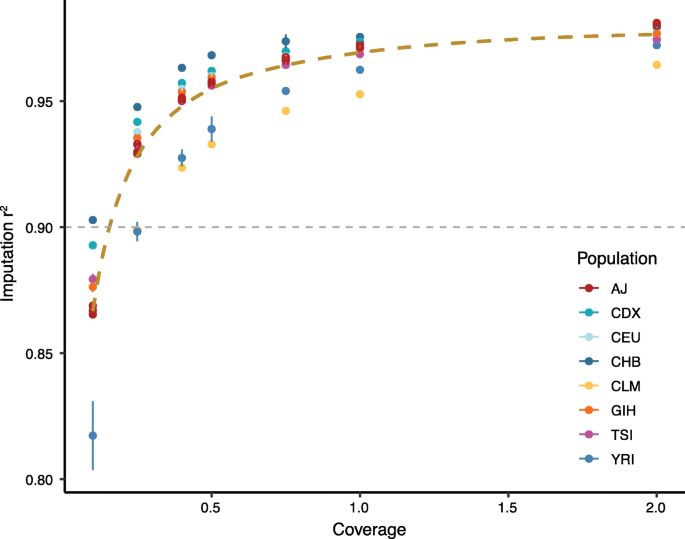
Background Inherited susceptibility to common, complex diseases may be caused by rare, pathogenic variants (“monogenic”) or by the cumulative effect of numerous common variants (“polygenic”). Comprehensive genome interpretation should enable assessment for both monogenic and polygenic components of inherited risk. The traditional approach requires two distinct genetic testing technologies—high coverage sequencing of known genes to detect monogenic variants and a genome-wide genotyping array followed by imputation to calculate genome-wide polygenic scores (GPSs). We assessed the feasibility and accuracy of using low coverage whole genome sequencing (lcWGS) as an alternative to genotyping arrays to calculate GPSs. Methods First, we performed downsampling and imputation of WGS data from ten individuals to assess concordance with known genotypes. Second, we assessed the correlation between GPSs for 3 common diseases—coronary artery disease (CAD), breast cancer (BC), and atrial fibrillation (AF)—calculated using lcWGS and genotyping array in 184 samples. Third, we assessed concordance of lcWGS-based genotype calls and GPS calculation in 120 individuals with known genotypes, selected to reflect diverse ancestral backgrounds. Fourth, we assessed the relationship between GPSs calculated using lcWGS and disease phenotypes in a cohort of 11,502 individuals of European ancestry. Results We found imputation accuracy r2 values of greater than 0.90 for all ten samples—including those of African and Ashkenazi Jewish ancestry—with lcWGS data at 0.5×. GPSs calculated using lcWGS and genotyping array followed by imputation in 184 individuals were highly correlated for each of the 3 common diseases (r2 = 0.93–0.97) with similar score distributions. Using lcWGS data from 120 individuals of diverse ancestral backgrounds, we found similar results with respect to imputation accuracy and GPS correlations. Finally, we calculated GPSs for CAD, BC, and AF using lcWGS in 11,502 individuals of European ancestry, confirming odds ratios per standard deviation increment ranging 1.28 to 1.59, consistent with previous studies. Conclusions lcWGS is an alternative technology to genotyping arrays for common genetic variant assessment and GPS calculation. lcWGS provides comparable imputation accuracy while also overcoming the ascertainment bias inherent to variant selection in genotyping array design.
)
Whole-genome sequencing Oxford Nanopore Technologies

Low-Pass Genome Sequencing: Validation and Diagnostic Utility from 409 Clinical Cases of Low-Pass Genome Sequencing for the Detection of Copy Number Variants to Replace Constitutional Microarray - ScienceDirect
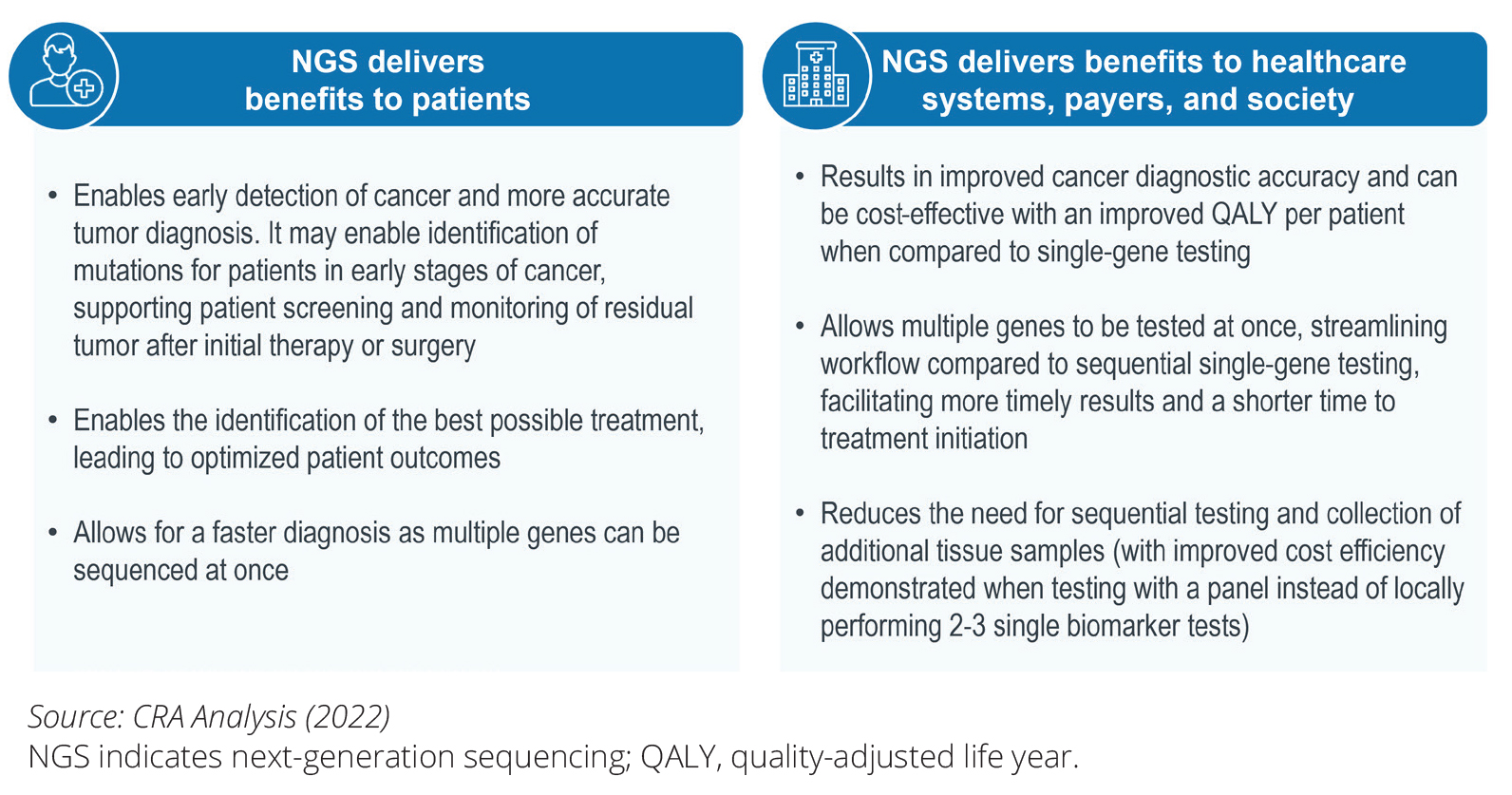
ISPOR - Accelerating Patient Access to Next-Generation Sequencing in Oncology: A Plan of Action
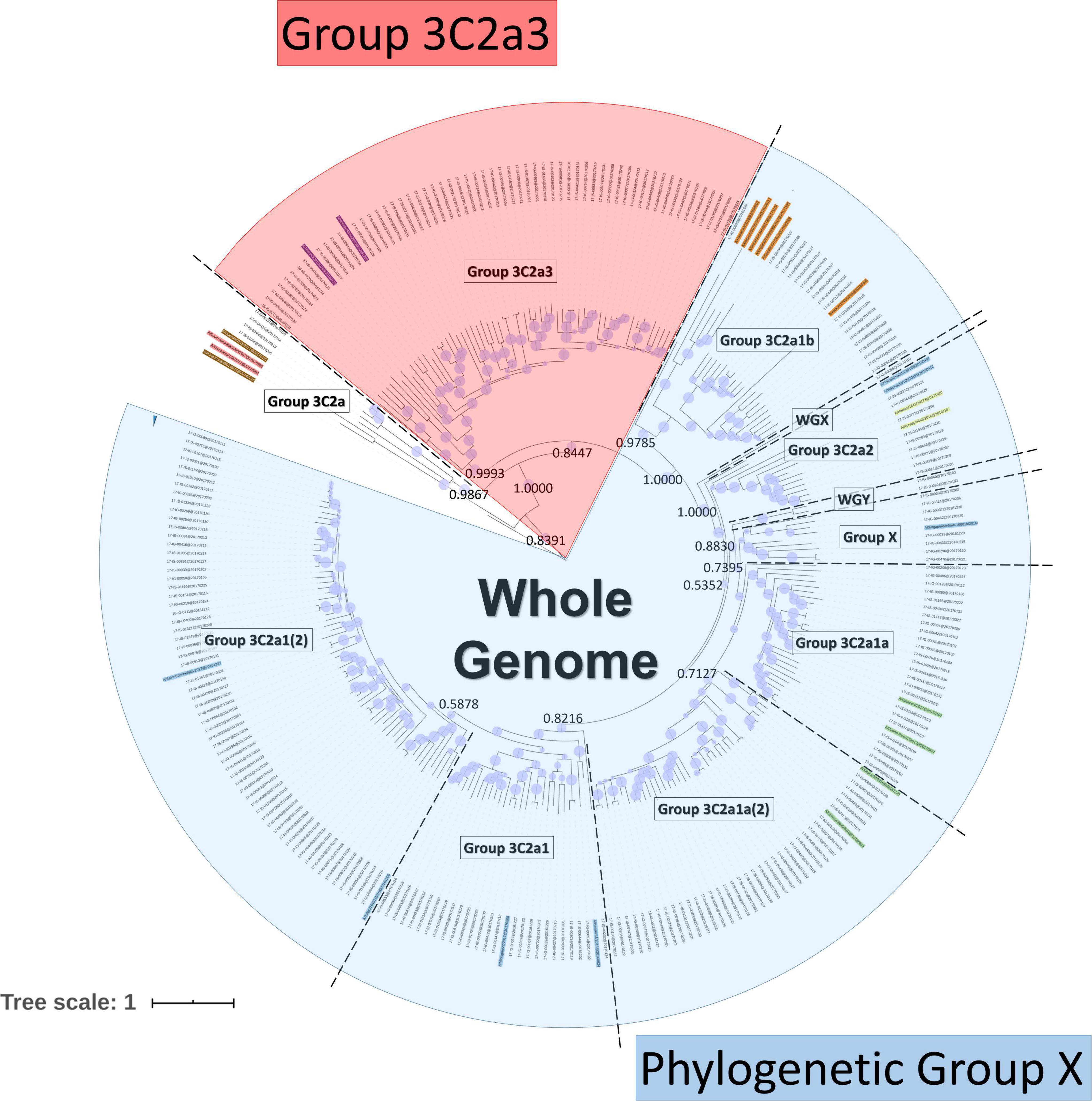
Frontiers Whole-Genome Sequence Approach and Phylogenomic Stratification Improve the Association Analysis of Mutations With Patient Data in Influenza Surveillance

Relative matching using low coverage sequencing
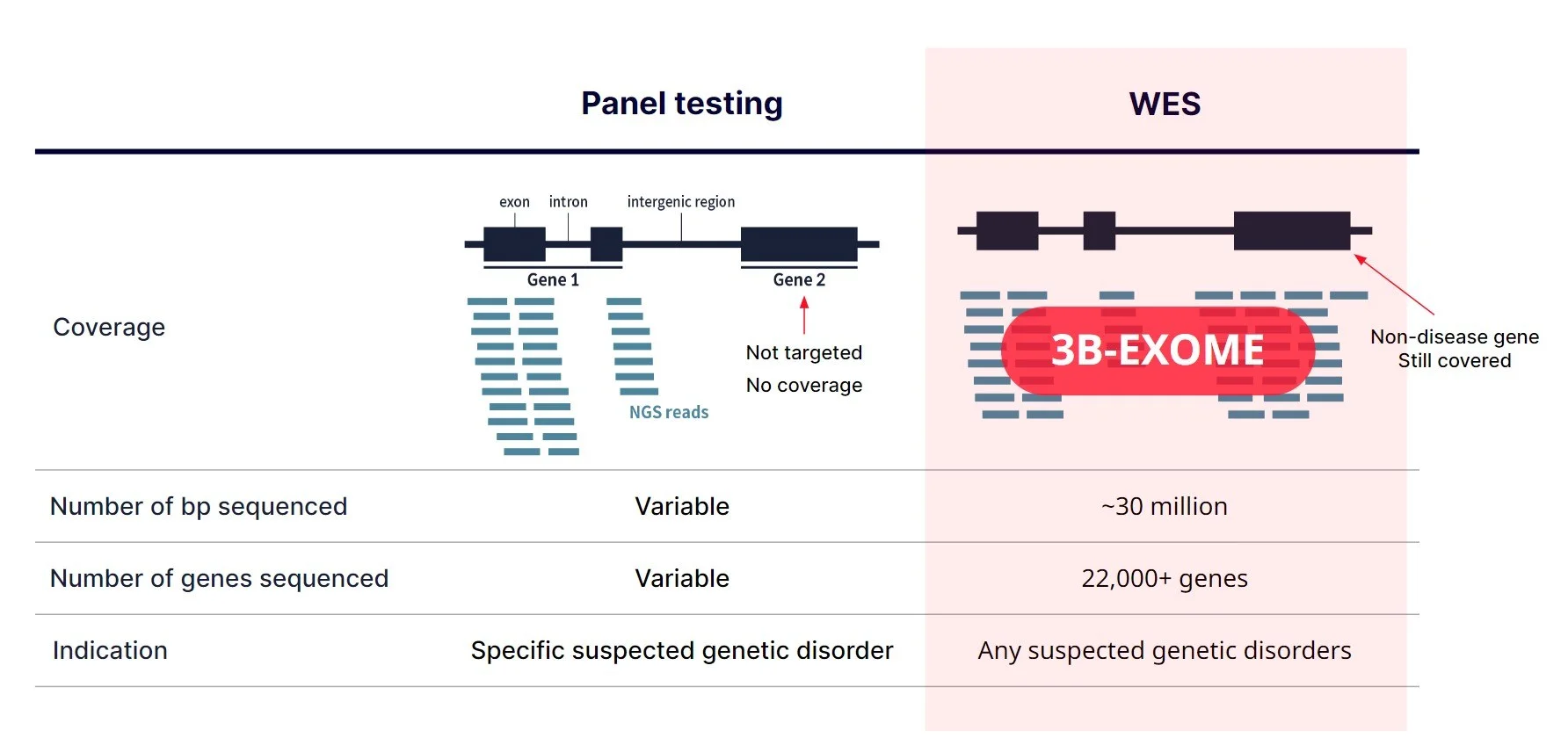
Sequencing Depth vs Coverage
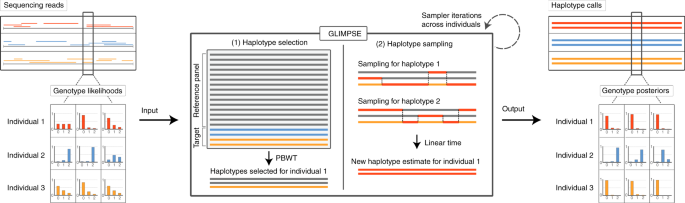
Efficient phasing and imputation of low-coverage sequencing data using large reference panels

PDF] Polygenic background modifies penetrance of monogenic variants conferring risk for coronary artery disease, breast cancer, or colorectal cancer
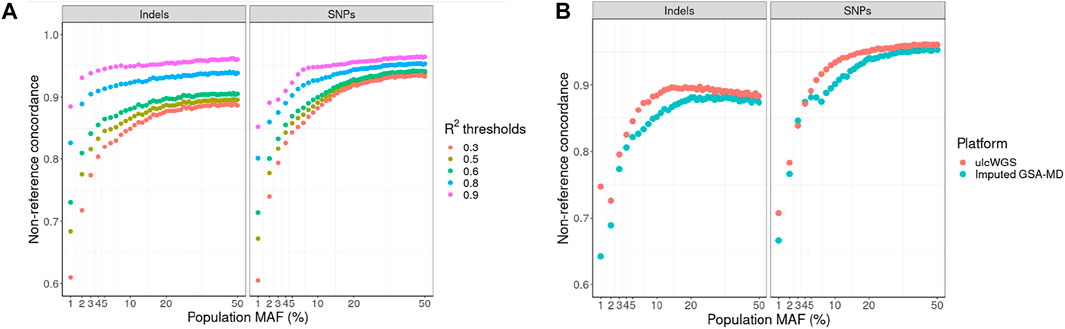
Frontiers Ultra Low-Coverage Whole-Genome Sequencing as an Alternative to Genotyping Arrays in Genome-Wide Association Studies






