Two closed bulbs of equal volume V containing an ideal gas initially at pressure Pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure

Two closed bulbs of equal volume V containing an ideal gas initially at pressure Pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure below. The temperature of one of the bulbs is then raised to T2. The final pressure pf is :
Two closed bulbs of equal volume V containing an ideal gas initially at pressure Pi and temperature T1 are connected through a narrow tube of negligible volume as shown in the figure below- The temperature of one of the bulbs is then raised to T2- The final pressure pf is -
Since the above system is a closed one, the total number of moles of the ideal gas will be equal before and after the temperature increase.
Hence in the given c

Mass transfer dr auroba

Two closed bulbs of equal volume (V) containing an ideal gas initially pressure p and temperature T. are connected through a narrow tube of negligible volume as shown in the figure below.


Chapter 1 INTRODUCTION AND BASIC CONCEPTS

Two closed containers of equal volume of air are initially at 1.05xx10
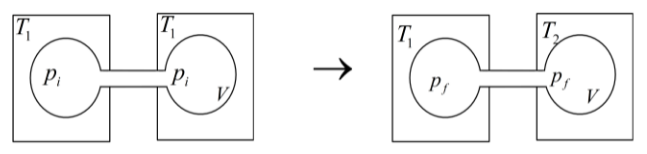
Two closed bulbs of equal volume (\[V\]) containing an ideal gas initially at pressure \[{p_i}\] and temperature \[{T_1}\] are connected through a narrow tube of negligible volume as shown in the figure

2.ideal Gas - Final, PDF, Gases
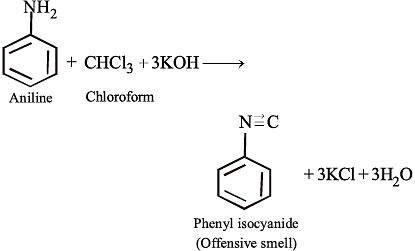
NEET Practice Test - 22 Free MCQ Practice Test with Solutions - NEET

Relating Pressure, Volume, Amount, and Temperature: The Ideal Gas Law

A one litre glass bulb is evacuated and weighed. The weight is 500 g.

Solved A 2. Two identical glass bulbs are connected by a







