
Click here:point_up_2:to get an answer to your question :writing_hand:the compression factor z for co at 7c and 100atm is 021 calculate the volume
Click here👆to get an answer to your question ✍️ The compression factor -Z- Co- 7-C and 100 atm is 0-21- Calculate the volume of a 4 mole sample of co- same temperature and pressure -use R - 0-08 L- atm-K-mol -1- 0-192 -2- 0-05 L -3- 0-38 L -4- 0-44 L closed container can be
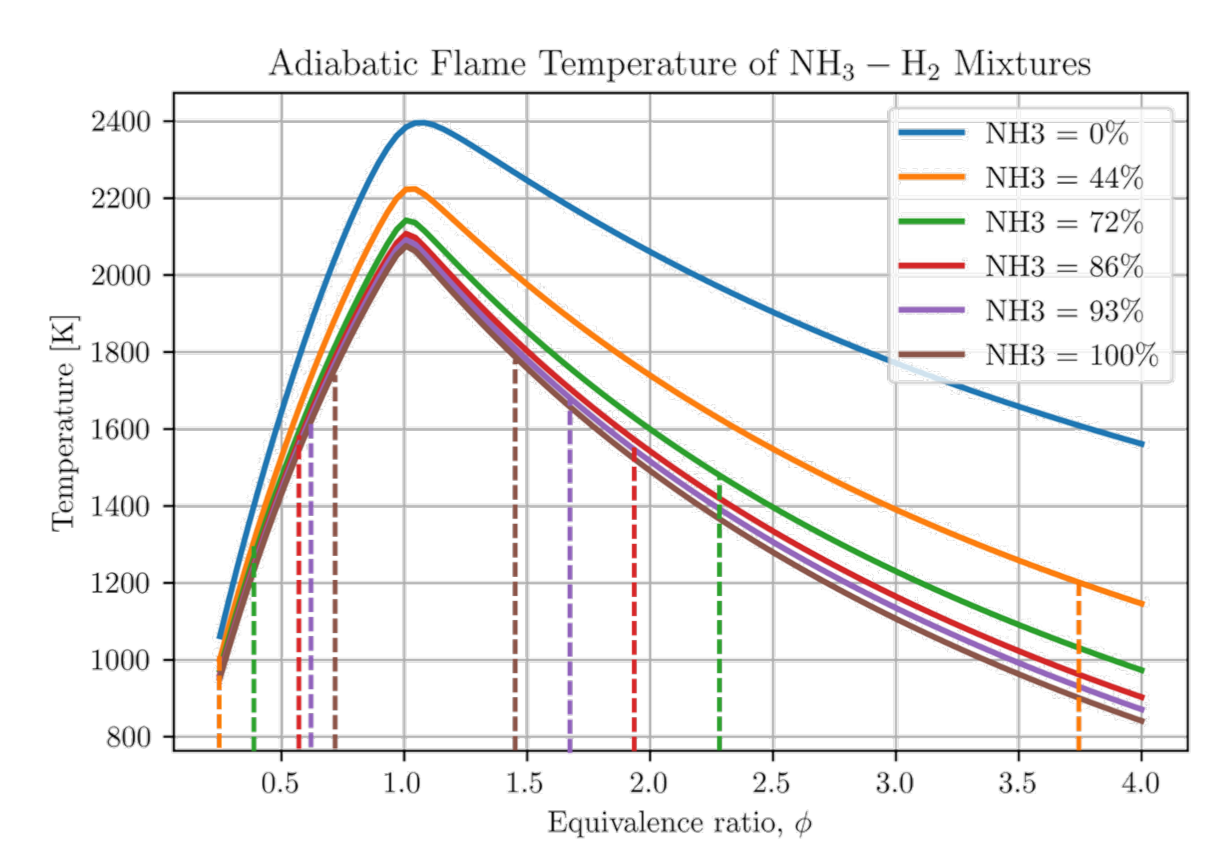
Energies, Free Full-Text

Sheet - 01 - Real Gas, PDF, Gases

CN108069946B - Substituted quinazoline compounds having the

The compression factor (Z) for CO2 at 7∘C and 100 atm is 0.21
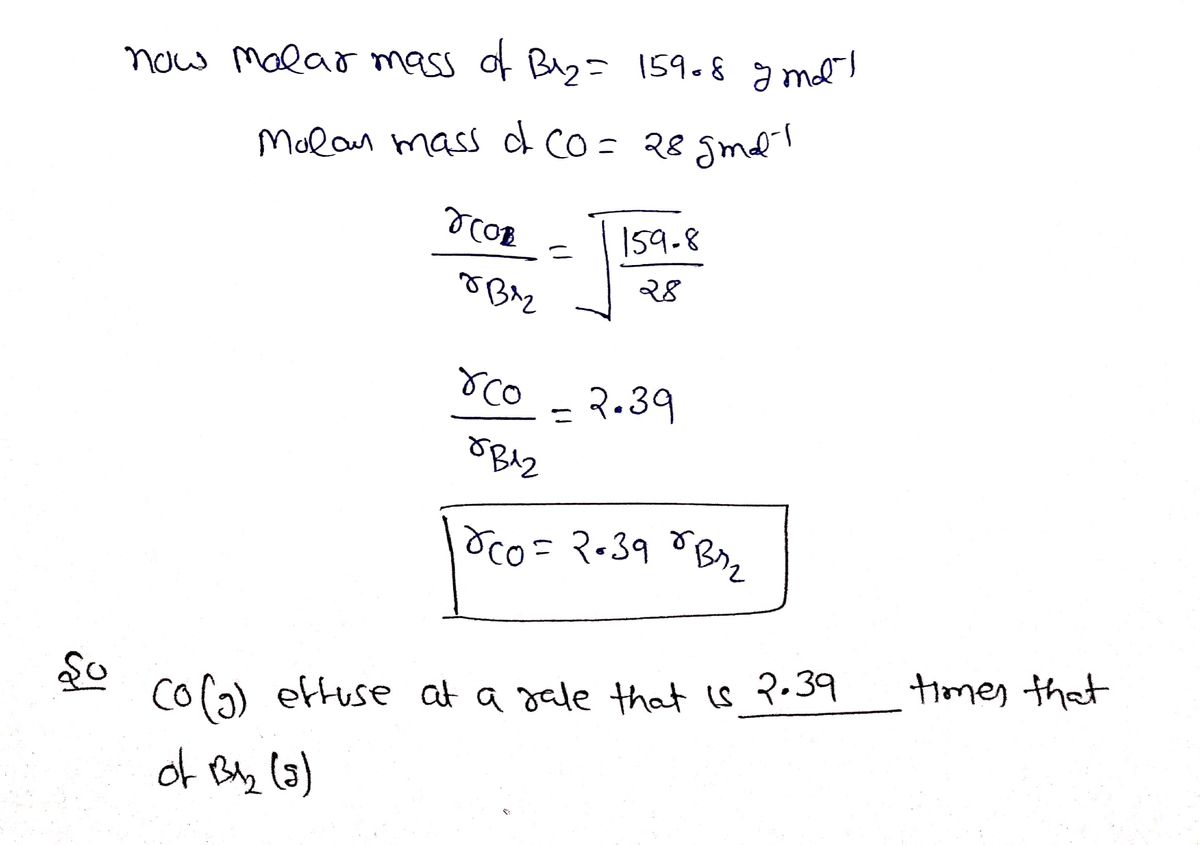
Answered: CO(g) effuses at a rate that is ______…

Chemical Thermodynamics

Answered: Calculate the molar volume of sulphur…

fundamentals of engineering supplied-reference handbook

Gas dynamics and jet propulsion – presentationof problemsanswers

SOLVED: Use the generalized correlation for the compressibility
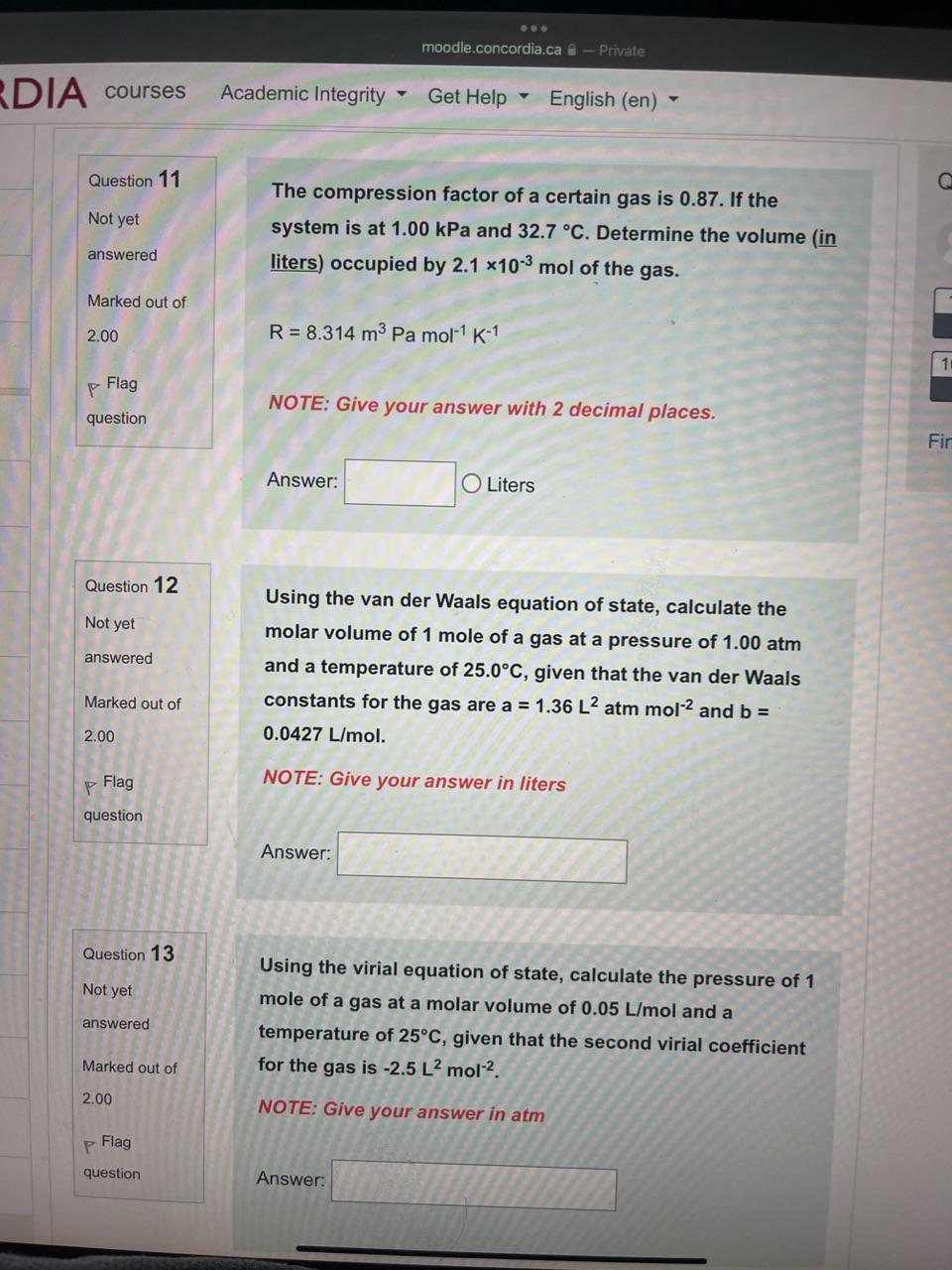
Solved Question 11 The compression factor of a certain gas

Modelling of metal hydride hydrogen compressors from







