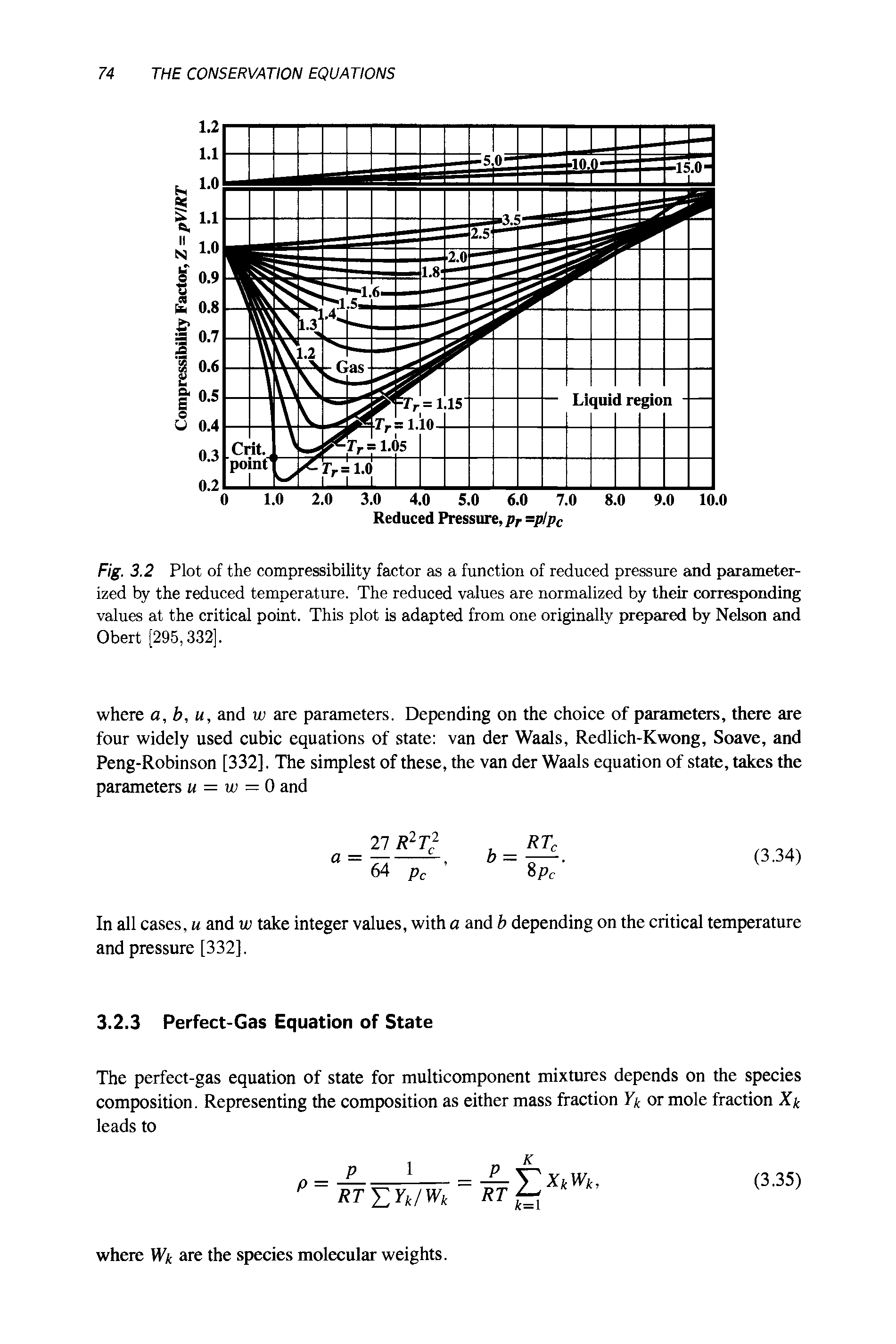
Compressibility factor Z = PV / nRT is plotted against pressure as

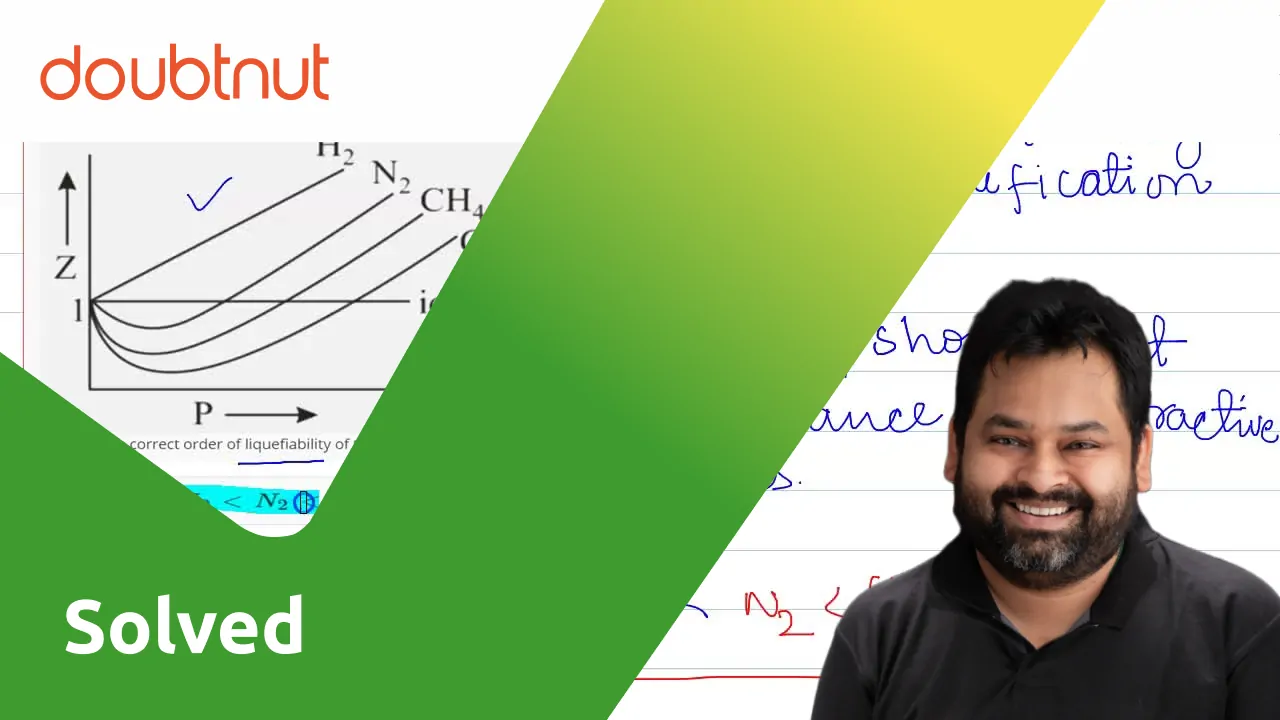
Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Deviation of Real Gases from Ideal Gas Behaviour - Chemistry for ACT PDF Download

The given graph represents the variation of Z (compressibility factor) vs. P three real gases A, B and C. Identify the correct statementFor the gas A, a=0 and its dependence on P

Gas Compressibility - an overview

Gas—General - ScienceDirect
1.5 Real Gases and the Virial Equation - Mail

Compressibility factor - Wikipedia

3.2 Real gas and compressibility factor – Introduction to Engineering Thermodynamics

Deviation Of Real Gas From Ideal Gas Behavior

Compressibility factor of water vapor along its saturation curve. Error
Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure