The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is (Enthalpy of vaporization for water is 40.63 kJ mol–1)
The entropy change for the conversion of 36 g water to vapour at its boiling point at 1 atm is -Enthalpy of vaporization for water is 40-63 kJ mol-1
Solubility - Wikipedia

559) Calculate the entropy change when 3.6 g of liquid water is completely converted into vanours 373 K. The molar heat of vaporization of water is 40.85 kJ mol! b) 2.189 JK

What is the correct method to convert volumetric flow rate of mixture to mass flow rate ? : r/thermodynamics

66. The entropy change for the conversion of 36 g of water to vapour at 100°C (Normal boiling point) is
The latent heat of vapourisation of water at 100 Celcius is 540 cal/g . Calculate the entropy increase when one mole of water at 100 Celcius is evaporated.
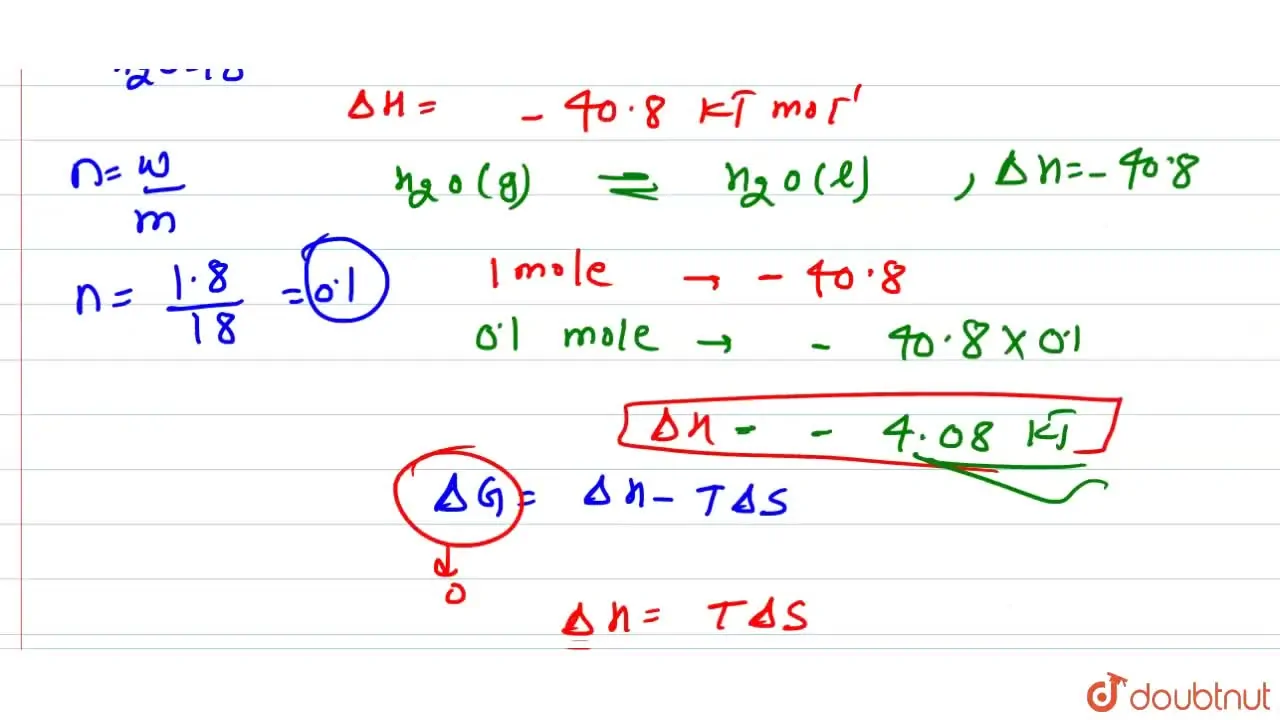
When 1.8 g of steam at the normal boiling point of water is converted

Calculate the entropy change the conversion of 1g ice to water 273K.[ Delta {H}_{fusion} =6.025kJ {mol}^{-1}]
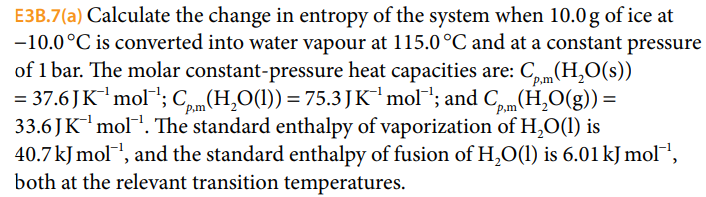
Solved E3B.7(a) Calculate the change in entropy of the

The entropy change associated with the conversion of 1 kg of ice 273 K to water vapours 383 K is : (Specific heat of water liquid and water vapour are 4.2 KJ

50. Ir Water vapour is assumed to be a perfect gus, molar enthalpy change vapourisation of 1 mole of water 1 bar and 100° C is 41 mol Calculate the internal energy
What is the entropy change in going from vapour to liquid state at any temperature? - Quora







