The vapour pressure of a solution having 2.0 g of solute X (gram atomic mass=32 g/mol) in 100 g of CS2 (vapour pressure =854torr) is 848.9 torr.The molecular formula of solute 1) X 2)X2 3)X4 4)X8
The vapour pressure of a solution having 2-0 g of solute X -gram atomic mass-32 g-mol- in 100 g of CS2 -vapour pressure -854torr- is 848-9 torr-The molecular formula of solute 1- X 2-X2 3-X4 4-X8

NCERT Solutions For Class 12 Chemistry Chapter 2 Solutions

14. The vapour pressure of the solution having 2.0 g of solute (a molecu the solution having 2.0 g of solute (a molecule of x with atomic ma = 32 g/mol) in
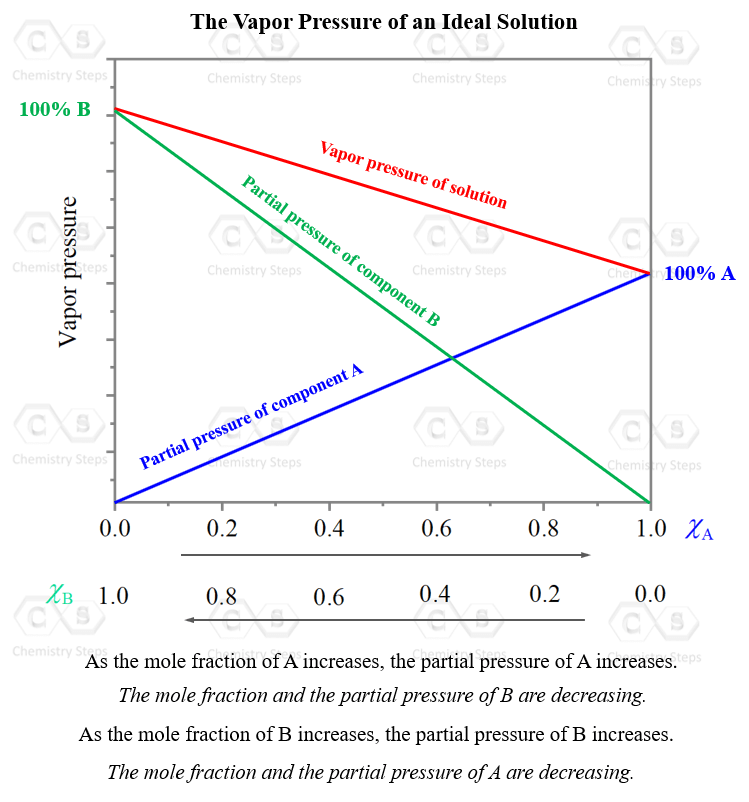
Vapor Pressure Lowering - Chemistry Steps

Telugu] The vapour pressure of a solution having 2.0g of a solute X (

13.6: Vapor Pressures of Solutions - Chemistry LibreTexts

Concentration of Solution: Definition, Formulas & Solved Examples

Molar Mass from Osmotic Pressure

Vapour Pressure - Definition, Raoult's Law, Formula & FAQs

Using the vapor-pressure curves in Figure 11.25, (d) estimate the

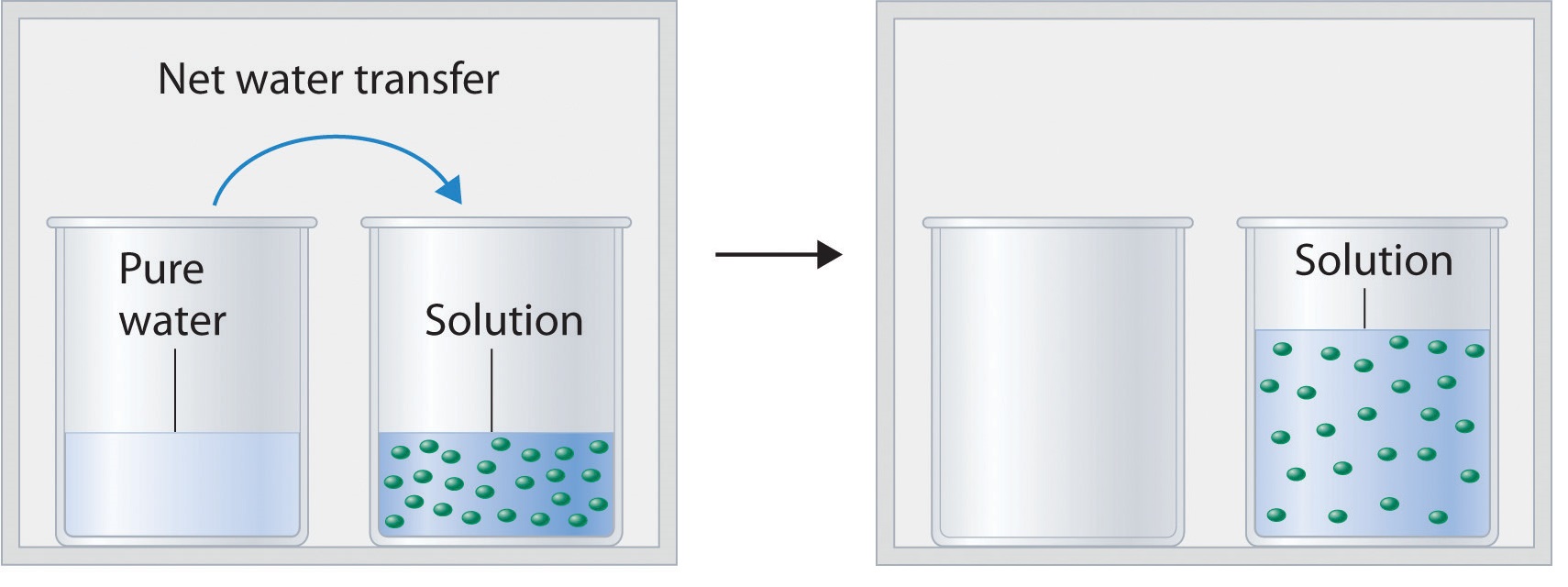
13.6: Vapor Pressures of Solutions - Chemistry LibreTexts
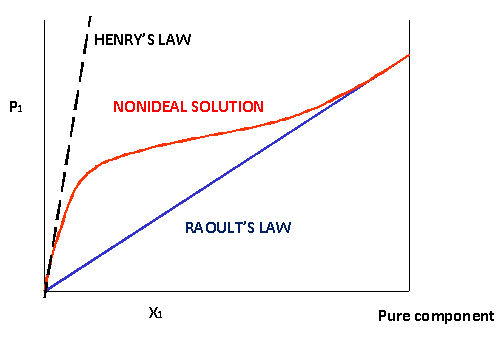
Raoult's Law - Chemistry LibreTexts

The vapour pressure of a solution having 2.0 g of a solute X( molar mass ..
Systems Thinking – CLUE: Chemistry, Life, the Universe and Everything