At high pressure, the compressibility factor 'Z' is equal toa


At high pressure, the compressibility factor 'Z' is equal toa

NEET Chemistry Chapter Wise Mock Test - Mock Test 2 - CBSE Tuts

Explain how the compression factor varies with pressure and
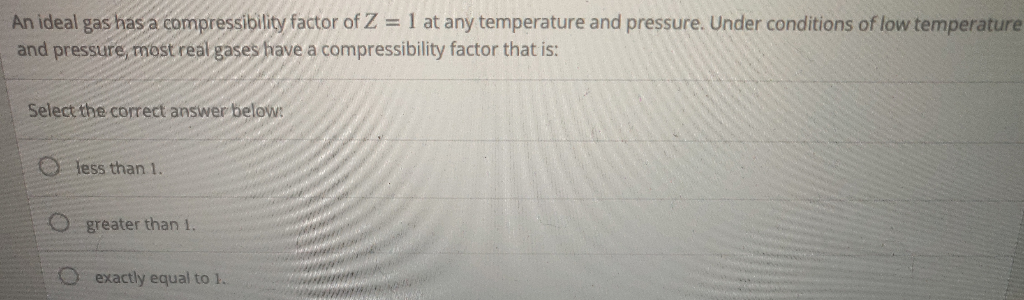
Solved An ideal gas has a compressibility factor of Z = 1 at

Gas compressibility factor Z: Ideal gas vs Real gas
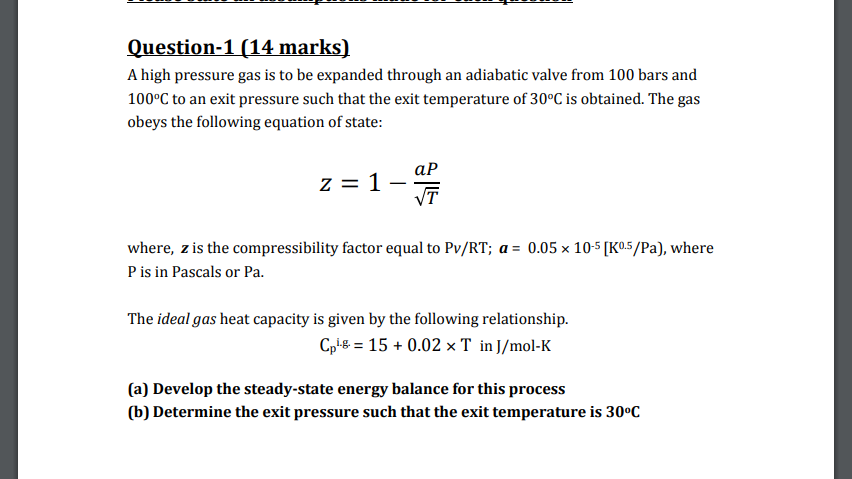
Solved A high pressure gas is to be expanded through an

At a high pressure, the compressibility factor (Z) of a real gas is us
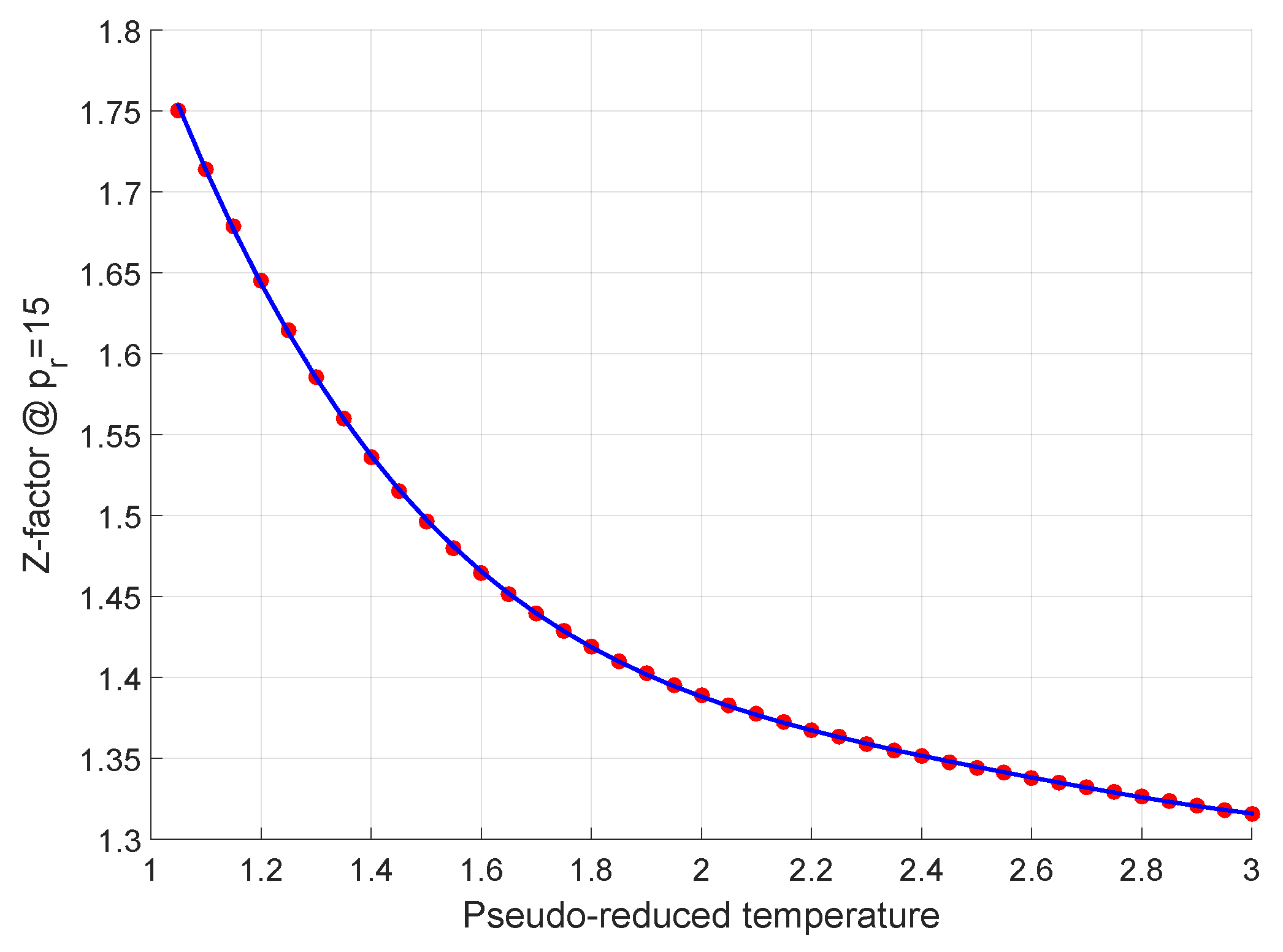
Energies, Free Full-Text

At very high pressure, the compressibility factor of one mole of a gas
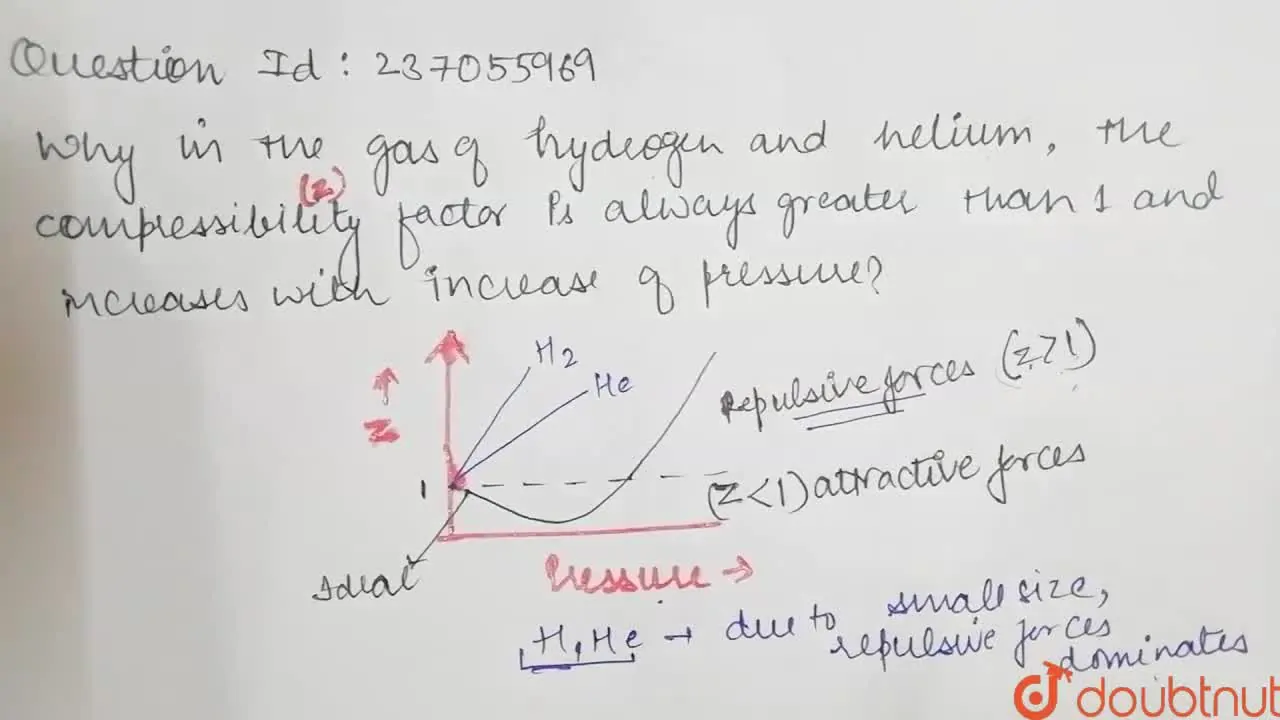
Why in the case of hydrogen and helium, the compressibility factor is
Is z (compressibility factor) vs P (pressure) graph drawn by changing volume? If it is why it isn't drawn by changing mole - Quora

What is the compressibility factor (Z) for 0.02 mole of a van der Waals's gas at pressure of 0

NEET Chemistry Chapter Wise Mock Test - Mock Test 2 - CBSE Tuts
Finding the compressibility factor (Z)

NEET Chemistry Chapter Wise Mock Test - Mock Test 2 - CBSE Tuts



:max_bytes(150000):strip_icc()/gerber-baby-3-pack-training-pants-94b2d1b5188e4b4ca90cfae5a479a869.jpg)

