Solved 1. A cup of hot 50°C water is poured onto 35g of ice

Answer to Solved 1. A cup of hot 50°C water is poured onto 35g of ice

SOLVED: A 35 g block of ice at -14°C is dropped into a calorimeter (of negligible heat capacity) containing 400 g of water at 0°C. When the system reaches equilibrium, how much
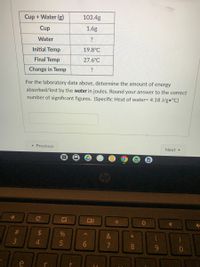
Answered: Cup + Water (g) 103.4g Cup 1.6g Water ?…

How heat Is Measured. Some specific heat capacity of substances at 25 0 C Substance Specific heat (J /g. 0 C) Water Aluminum Copper ppt download

single serve dessert with 19 grams of quality protein 👇👇 🍫I put my

An ice cube whose mass is 50 g is taken from a refrigerator where its temperature was`-10^C`. If

ConcepTest Clicker Questions - ppt download

Recipes – World AeroPress Championship

Protein Ninja Creami Icecream - Lemon8 Search

SOLVED: A cup of hot 50°C water is poured onto 35g of ice at 0°C until the entire system ends up at 20°C. How much energy is needed to melt the 35g







