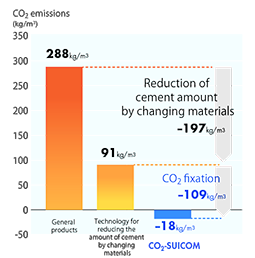
What is the change in internal energy (in J) of a system that

Description
I found an increase of 3100J Have a look

For a system that has equally spaced non-degenerate energy levels

Thermochemistry Chapter ppt download

Solved Be sure to answer all parts. What is the change in

Solved Be sure to answer all parts. What is the change in

When a system changes from $A$ to $B$ along the path shown o
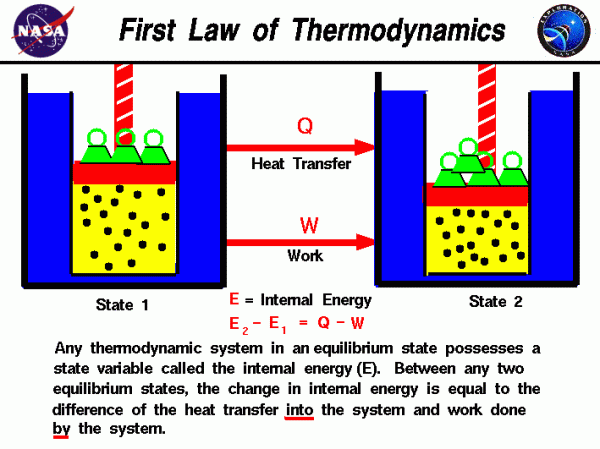
First Law - Internal Energy, Glenn Research Center

Answered: What is the change in internal energy…
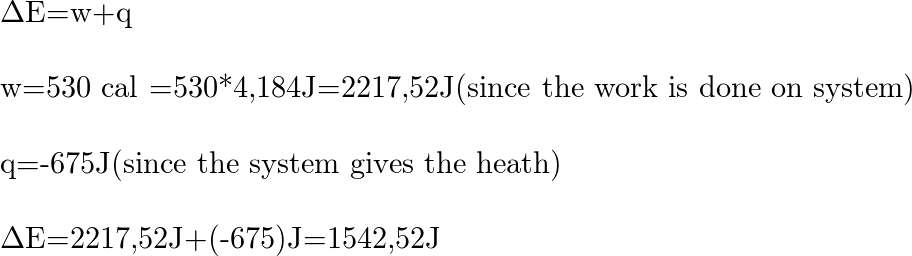
What is the change in internal energy (in J) of a system tha
What is the change in internal energy of a system that absorbs 455 J of heat and does 325 J of work? - Quora

If change in internal energy is given by 120 J. The work done by the system is 280 J. Calculate the heat associated with the process.A. 160 JB. 160 JC. 400 JD. 400 J
Related products
$ 15.50USD
Score 4.5(196)
In stock
Continue to book
$ 15.50USD
Score 4.5(196)
In stock
Continue to book
©2018-2024, paramtechnoedge.com, Inc. or its affiliates