
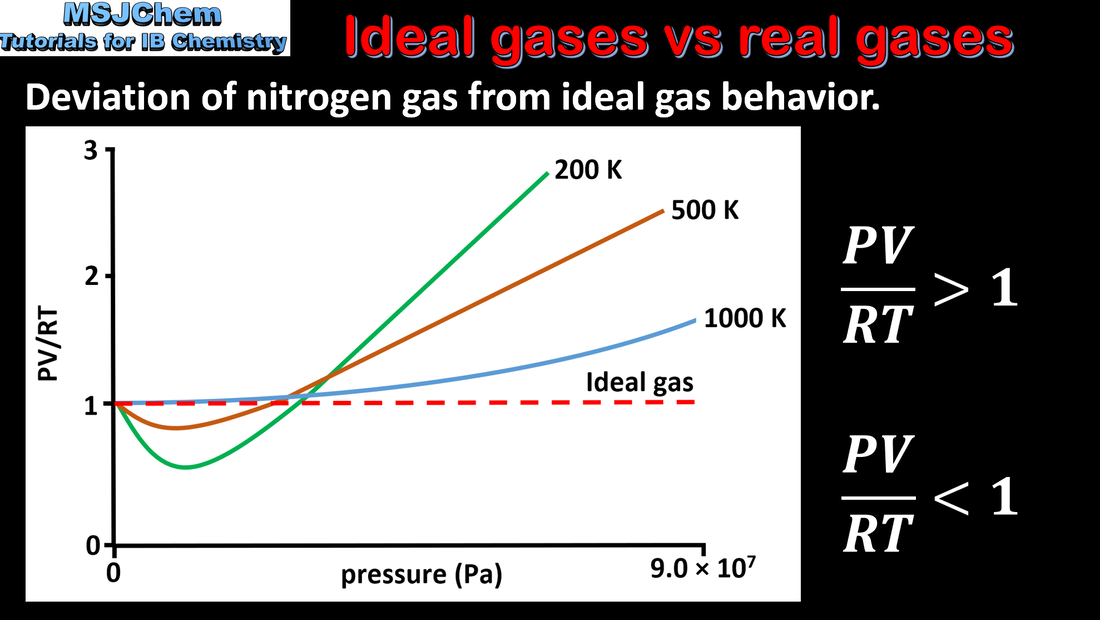
I am a bit confused (might be due to some conceptual misunderstanding) as to why doesn't Helium behave as an ideal gas (it shows a deviation from the $pV$ vs $p$ graph)? (Given the fact that it is

Kinetic Theory: Atomic and Molecular Explanation of Pressure and Temperature
PPT - KINETIC – MOLECULAR THEORY OF GASES PowerPoint Presentation, free download - ID:4176699
PPT - Gas Laws PowerPoint Presentation, free download - ID:210554
12.1: Introduction - Physics LibreTexts

Fast atom effect on helium gas/graphite interfacial energy transfer - ScienceDirect

NCERT Solutions for Class 11 Physics Chapter 13 Kinetic Theory

Ideal gas law - Wikiversity

Physical Behavior of Matter: Reference Table: Tables A, H, and T - ppt download

Kinetic Theory of Gases, PDF, Gases








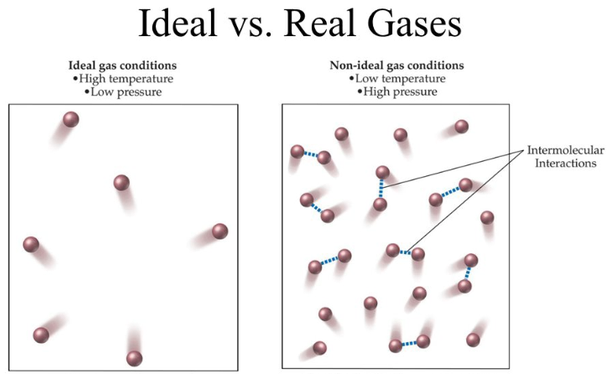

:max_bytes(150000):strip_icc()/200175879-001-56a12e6b5f9b58b7d0bcd67f.jpg)