32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32. 80 g of h2 is reacted with 80 g of o2 to form water. find out the mass of water obtained.which substance is the limiting reagent.
32- 80 g of h2 is reacted with 80 g of o2 to form water- find out the mass of water obtained-which substance is the limiting reagent

80 g of h2 is reacted - Chemistry - Chemical Kinetics - 14366697
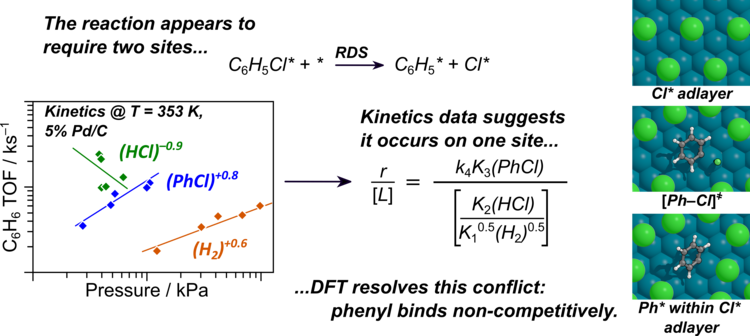
Hibbitts Group Publications

52. 80 g of H, is reacted with 80 g of O, to form water. Find out the mass of water obtained. Which substance is the limiting reagent?

10 g of hydrogen is burnt in the presence of excess oxygen. The mass of water formed is 90 g45 g10 g18 g

80 gram of H2 is reacted with 80 gram of O2 to form water find out the mass of water obtained which

Iit Mole Concept Questions, PDF, Chemical Compounds
If 10 cm3 of each hydrogen and oxygen gases react to form water, what will the limiting reagent be? - Quora
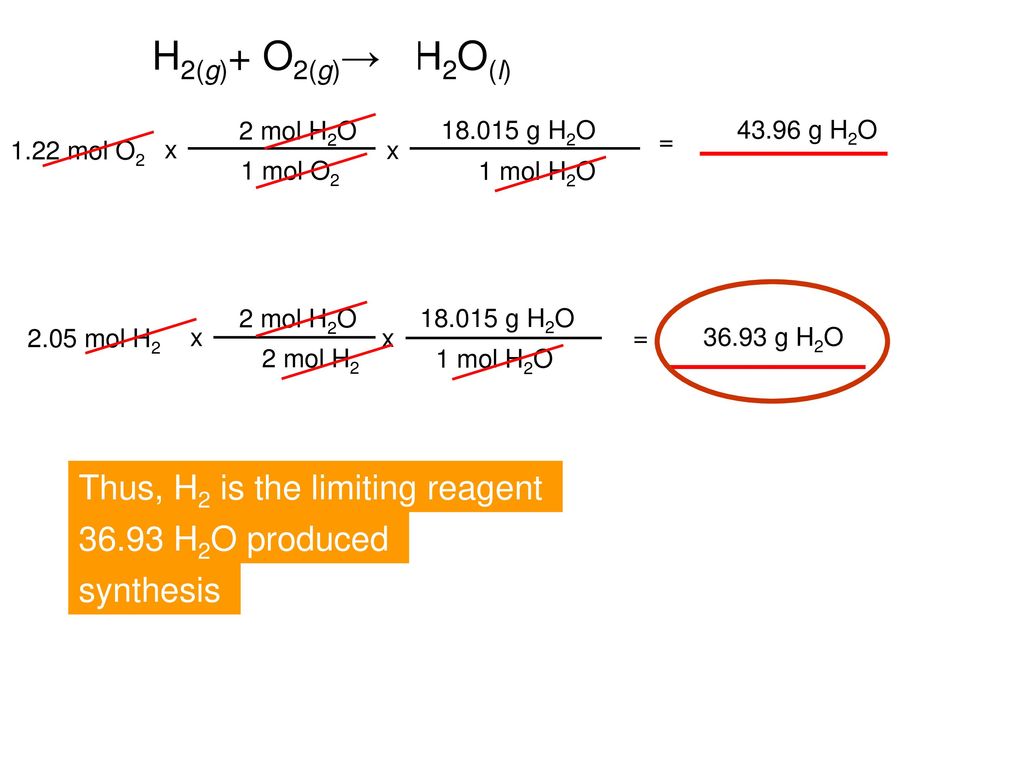
2H2(g)+ O2(g)→ 2H2O(l) Thus, H2 is the limiting reagent - ppt download

PPT - Topic 1.3 - Formulae, equations and amounts of substance PowerPoint Presentation - ID:5879234


)
,aspect=fit)

