the equation of state of a gas is p(v-nb)=rt where b and r are consta - askIITians

the equation of state of a gas is p(v-nb)=rt where b and r are constants. if the pressure and temperature are such that vm=10b what is the value of compressibi
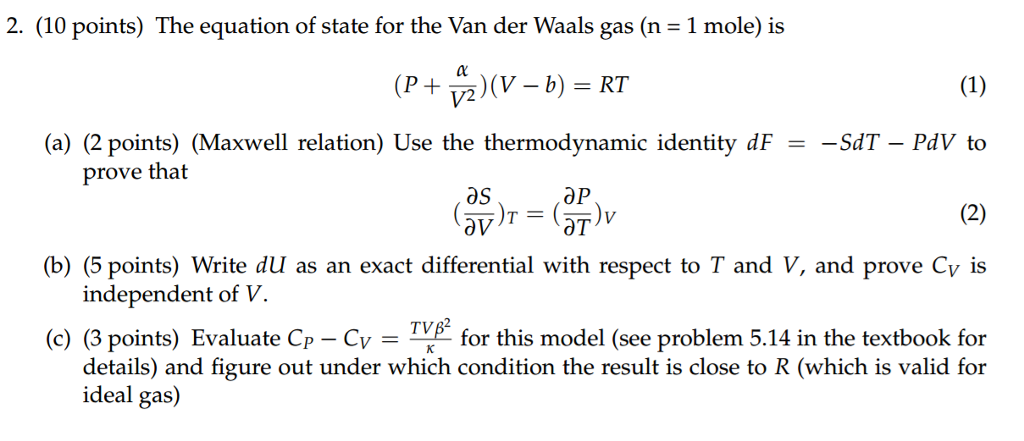
Solved The equation of state for the Van der Waals gas (n =

Revision Notes on Kinetic Theory of Gases

Class – 11. Notes States of matter – ADITYA CAREER INSTITUTE
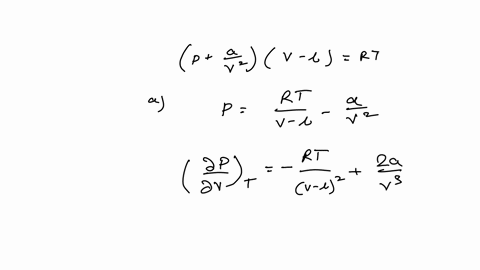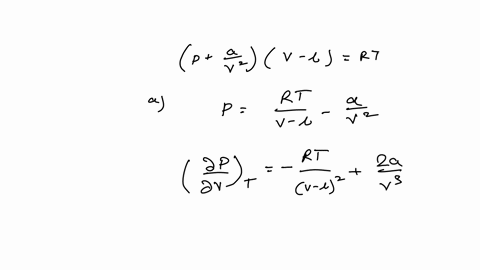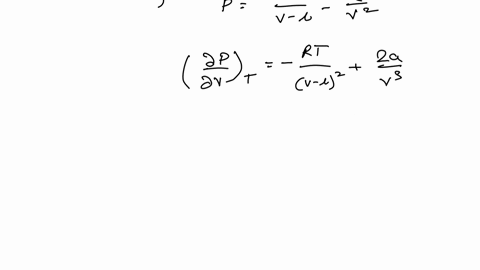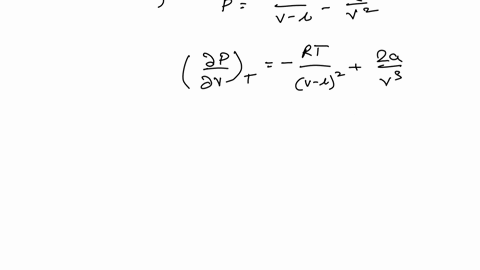
⏩SOLVED:The equation of state of an ideal gas is P V=n R T, where n…

Example 15 The equation of a state of a real gas is given by P +- (V - b) = RT, where T is absolute temperature, P is pressure, V is volume
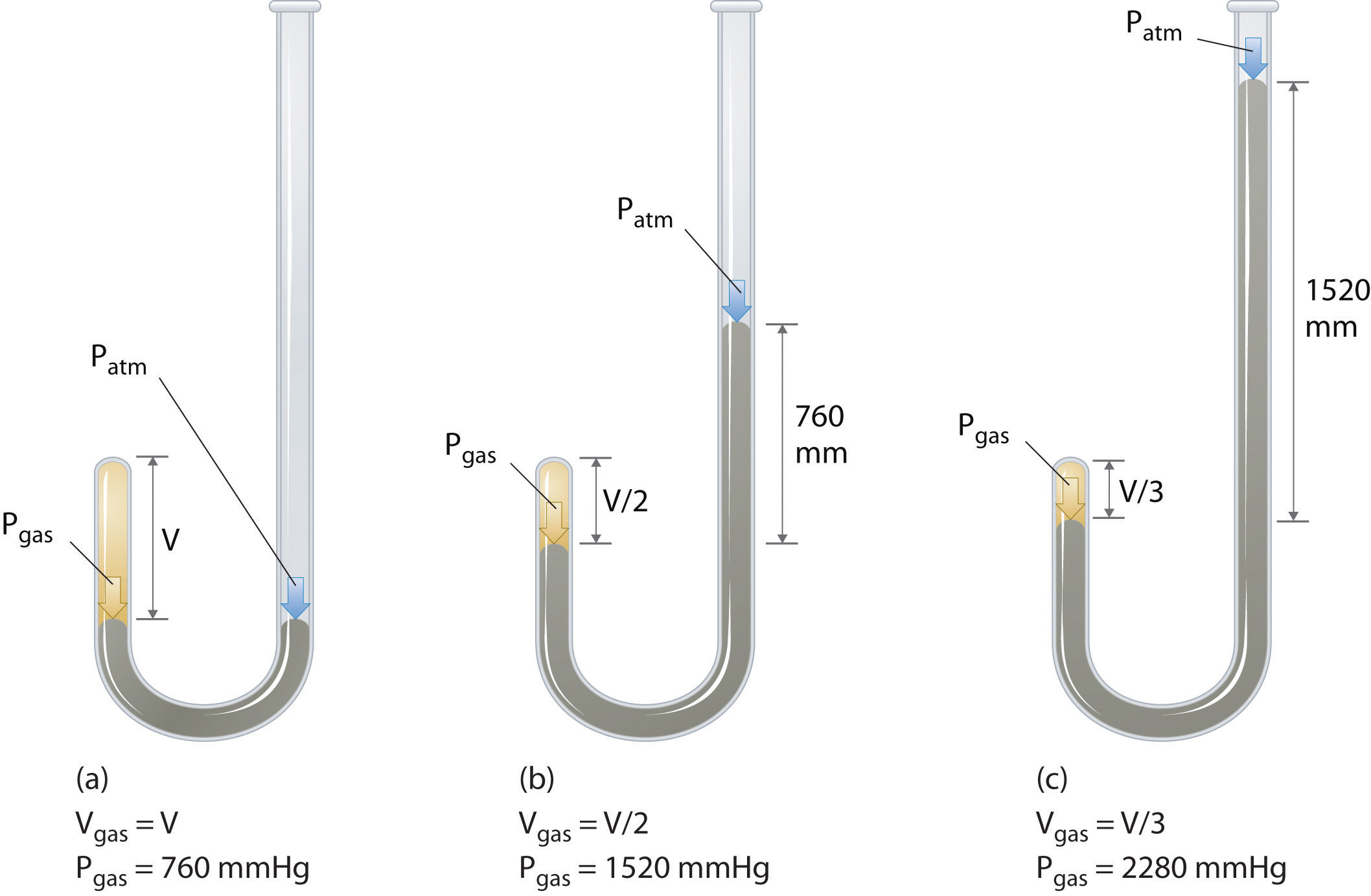
Gases

A gas obeys the equation of state `P(V-b) =RT` (The parameter b is a constnat The

Deviation From Ideal Gas Behavior - Study Material for IIT JEE
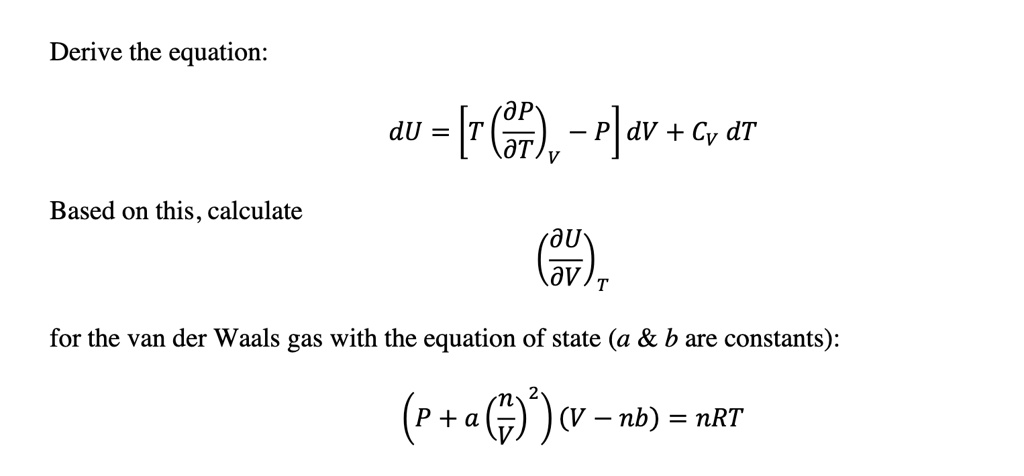
SOLVED: Derive the equation: dU = (6v - P)av + Cv dT Based on this, calculate for the van der Waals gas with the equation of state (a b are constants): (p +







