Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2

Compressibility factor Z = PV / nRT is plotted against pressure as shown below:What is the correct order for the liquefiability of the gases shown in the above graph? A. CO 2
Compressibility factor Z - PV - nRT is plotted against pressure as shown below-What is the correct order for the liquefiability of the gases shown in the above graph- A- CO 2- CH 4- N 2- H 2B- H 2- CH 4- N 2- CO 2C- CH 4- H 2- N 2- CO 2D- H 2- N 2- CH 4- CO 2

Compressibility Factor Z Important Concepts and Tips for JEE Main

Compressibility factor - Wikipedia

Gaseous State : Vander Waal Gas Equation - The Chemistry Guru

Bansal classes chemistry study material for iit jee by S.Dharmaraj - Issuu

gaseous state
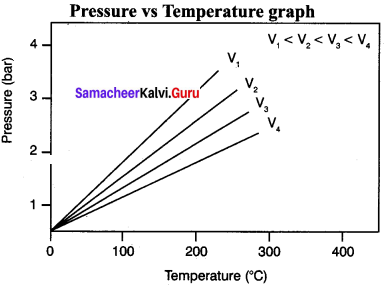
Samacheer Kalvi 11th Chemistry Solutions Chapter 6 Gaseous State – Samacheer Kalvi

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

Arihant Chemistry Sample Paper Class 11 by KnowledgeTest - Issuu

3.2 Real gas and compressibility factor – Introduction to
Why does gas liquefy at high pressure? Even at high-pressure

01 Gaseous State#### PDF, PDF, Gases
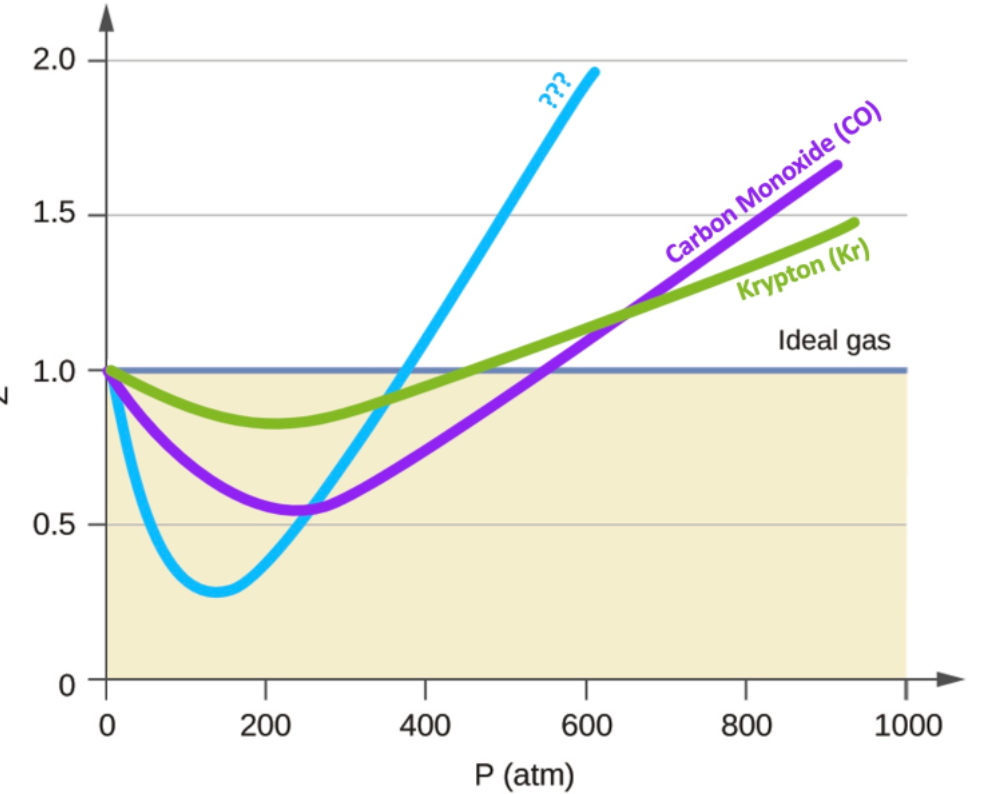
Solved Below is a plot of the compressibility factor (Z) as

gaseous state

Gas compressibility factor Z: Ideal gas vs Real gas

Chapter 3 - Physical Properties of Fluids: Gas Compressibility Factor







