Breaking local symmetry—why water freezes but silica forms a glass

Everyone knows that water freezes at 0 degrees C. Life on Earth would be vastly different if this were not so. However, water
Everyone knows that water freezes at 0 degrees C. Life on Earth would be vastly different if this were not so. However, water's cousin, silica, exhibits wayward behavior when cooled that has long puzzled scientists.
How does surface energy affect the rate of crystallization of a magma melt? - Quora
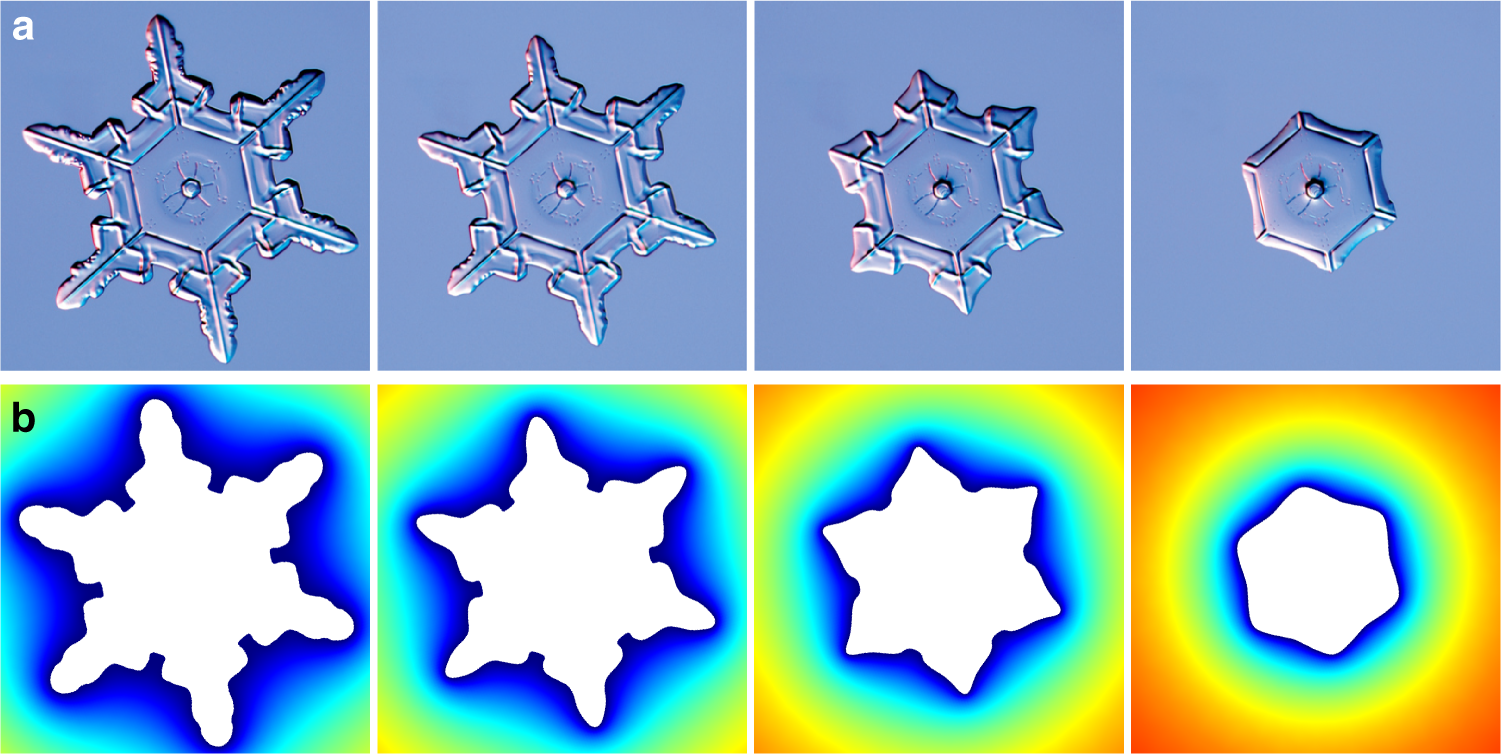
Singular sublimation of ice and snow crystals

Models of substructures participating in the silica network and
U Tokyo – sciencesprings

Structure and dynamics of nanoconfined water and aqueous solutions
Does water become less dense as it becomes colder or only when it reaches freezing temperature? - Quora

Ice Crystallization in Shear Flows The Journal of Physical Chemistry C

Freezing life within refractory, amorphous silicon dioxide
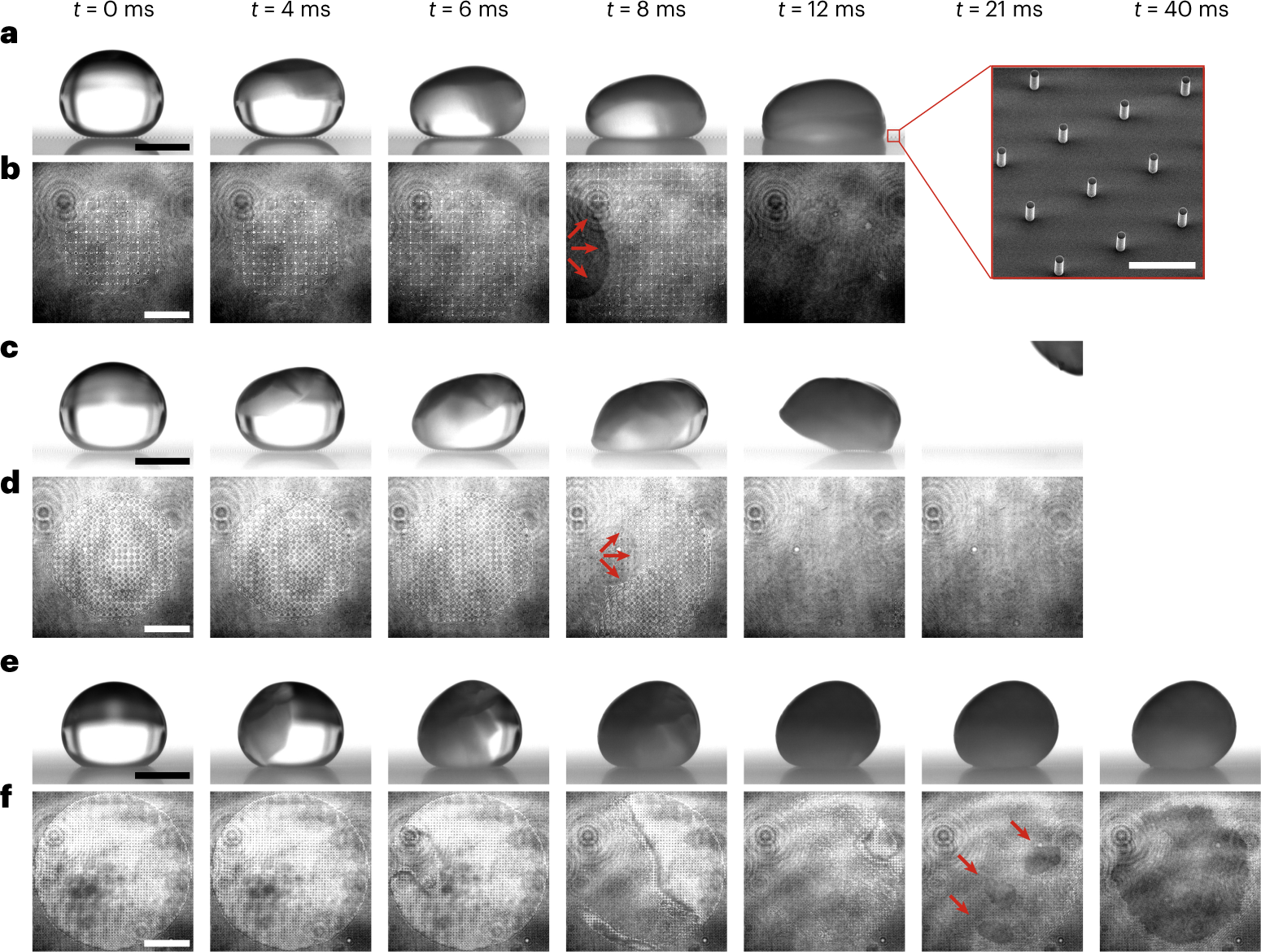
Freezing-induced wetting transitions on superhydrophobic surfaces

Two ice growth modes on hydrophilic and hydrophobic surfaces. (A) A

From Science Node: “The 5 fastest supercomputers in the world

The shapes of water: New research details water's mysterious phase transitions

Bendability of silicate glasses. a) Three‐point‐bending of a chemically






