Predicting ion diffusion from the shape of potential energy landscapes, Materials Chemistry, ChemRxiv

We present an efficient method to compute diffusion coefficients of multi-particle systems with strong interactions directly from the geometry and topology of the potential energy field of the migrating particles. The approach is tested on Li-ion diffusion in crystalline inorganic solids, predicting Li-ion diffusion coefficients within one order of magnitude of molecular dynamics simulations at the same level of theory while being several orders of magnitude faster. The speed and transferability of our workflow make it well suited for extensive and efficient screening studies of crystalline solid-state ion conductor candidates and promise to serve as a platform for diffusion prediction even up to density functional level of theory.

ACS Symposium Series (ACS Publications)
York Lab

Why Do Liquids Mix? The Mixing of Protic Ionic Liquids Sharing the Same Cation Is Apparently Driven by Enthalpy, Not Entropy
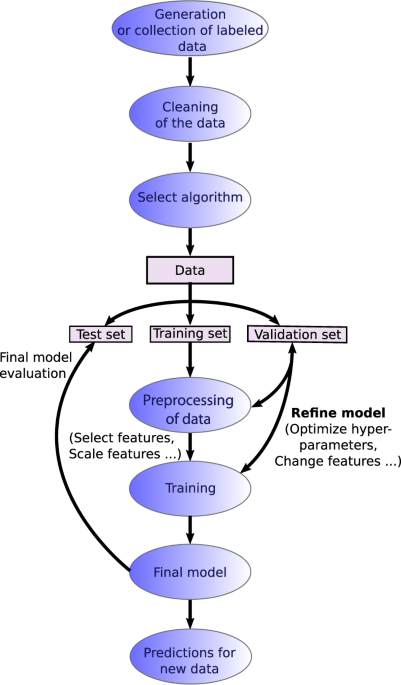
Recent advances and applications of machine learning in solid

PDF) The impact of alkali‐ion intercalation on redox chemistry and
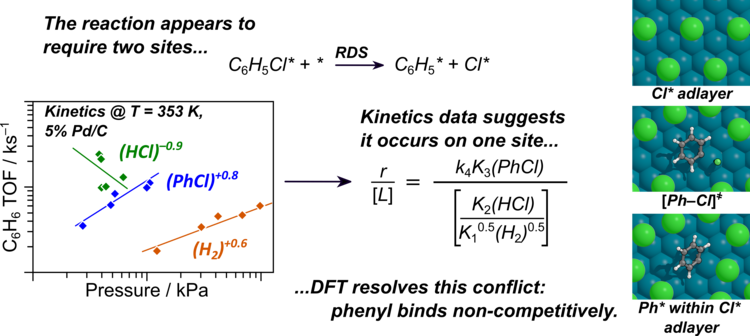
Hibbitts Group Publications

Evidence for a Solid-Electrolyte Inductive Effect in the Superionic Conductor Li10Ge1-xSnxP2S12. - Abstract - Europe PMC
OpenKIM · SNAP ZuoChenLi 2019 Si MO_869330304805_000 MO_869330304805 · Interatomic Potentials and Force Fields

Classical and reactive molecular dynamics: Principles and

ACS Symposium Series (ACS Publications)

Qianxiang Ai (@QaiAlex) / X

3 Measurement and Control of Molecular Quantum Systems Advancing Chemistry and Quantum Information Science: An Assessment of Research Opportunities at the Interface of Chemistry and Quantum Information Science in the United
IJMS, Free Full-Text

The Many-Body Expansion for Aqueous Systems Revisited: II. Alkali











