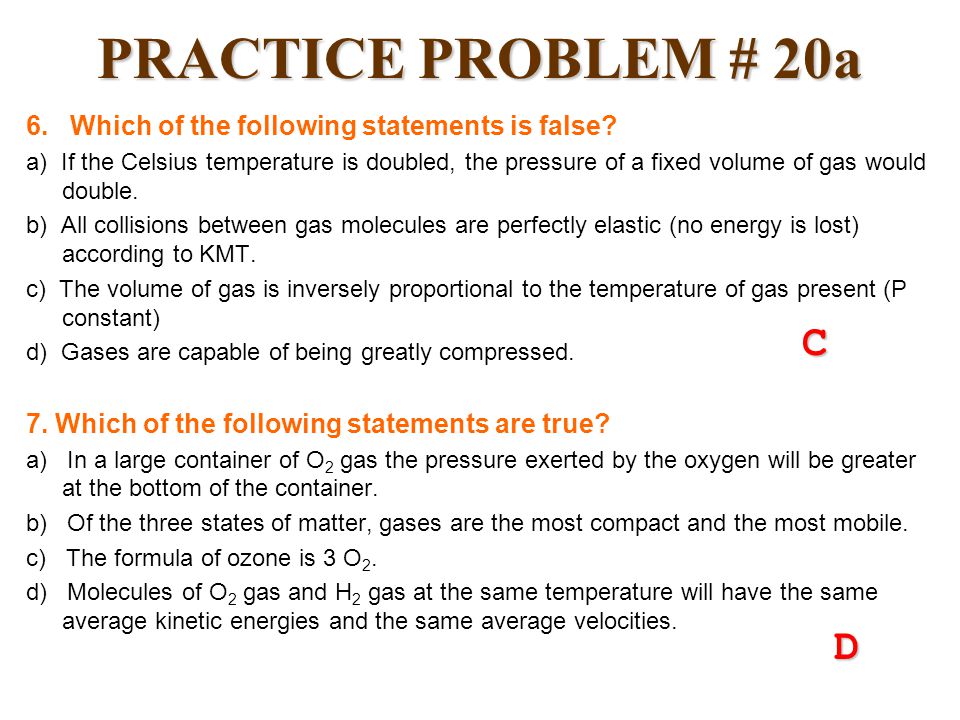
Which of the following statements is/are correct? (a) all real gases are less compressible

Which of the following statements is/are correct? (a) all real gases are less compressible than ideal gas at high pressures? (6) hydrogen and helium are more co
Seeing the Unseen Air is invisible, but we know it exists: Winds blow. - ppt video online download

For A Real Gas At 25∘C Temperature And High Pressure (99, 59% OFF

PVT Correlations with Python
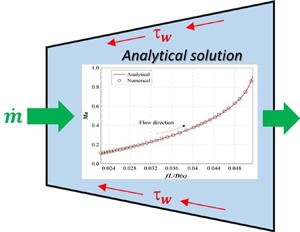
Exact solutions for quasi-one-dimensional compressible viscous flows in conical nozzles, Journal of Fluid Mechanics

What Are the Phases of Matter? — Overview & Examples - Expii

The curve of pressure volume PV against pressure P of the gas at a particular temperature is as shown, according to the graph which of the following is /are incorrect in the

SOLVED: Which of the following statements is true for real gases

Liquid - Wikipedia
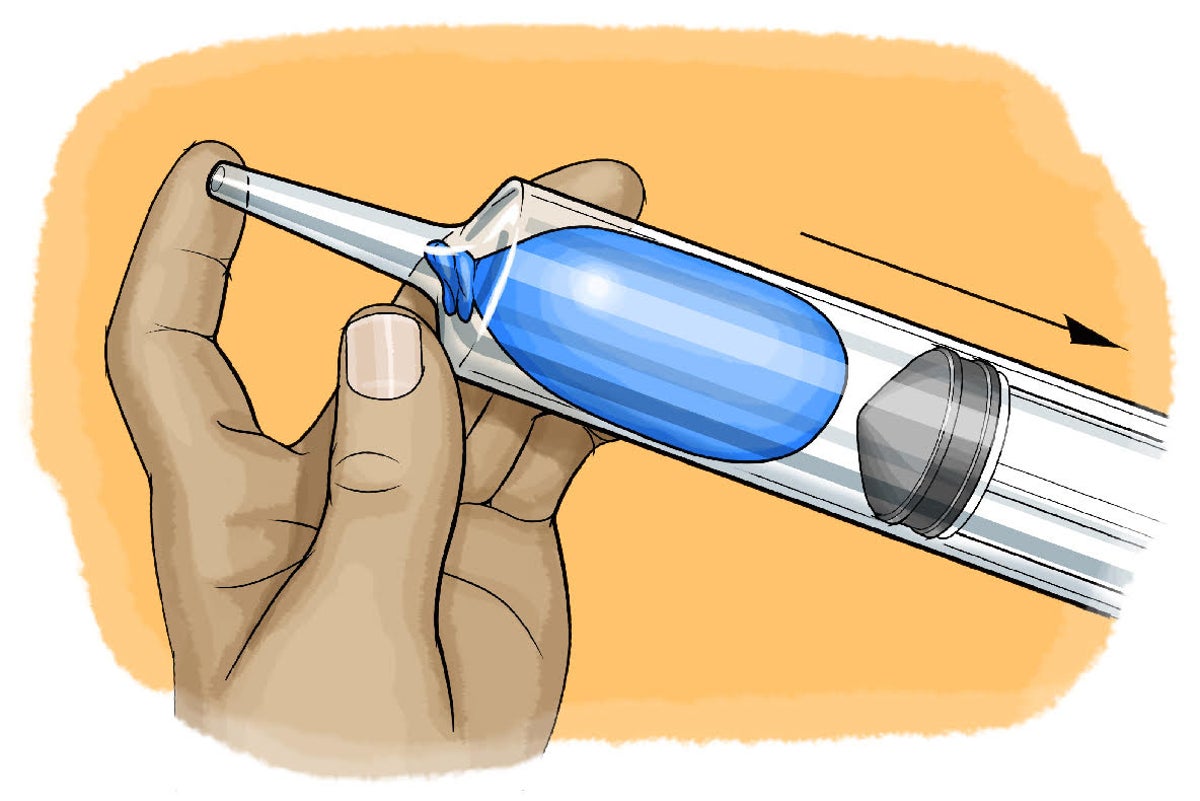
In and Out: Demonstrating Boyle's Law
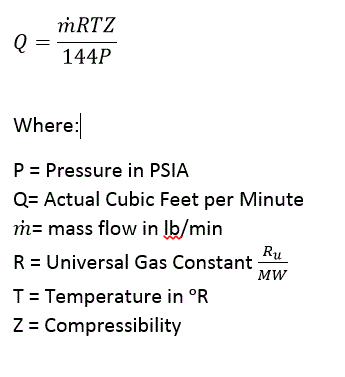
Volume and Mass Flow Calculations for Gases

Solved Which of the following statements about the

Consider the equation, Z=dfrac{PV}{nRT}. Which of the following statements is correct?When Z>1, real gases are easier to compress than the ideal gasWhen Z>1, real gases are difficult to compressWhen Z=1, real gases
Gaseous State (ADV) Question Paper











