20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20.If Z is a compressibility factor, van der Waals equation at low pressure can be written as
20-If Z is a compressibility factor- van der Waals equation at low pressure can be written as

a) Compressibility factor Z obtained from the Lee-Kesler EoS, and

Objectives_template

Which of these are correct? A) Z, compressibility factor, low pressure can be written as z = B) Z, low pressure can be written as z = 1 + P C) Z

Van der Waals equation, when pressure correction is ignored, one mole can be written as P(V - b) = RT. The correct expression compressibility factor will be

Deviations from ideal gas behaviour, intermolecular forces, Van der Waals equation of state, compressibility factors and the critical pressure and critical temperature of a gas revision notes doc brown's chemistry UK advanced

Cubic equations of state - Wikipedia
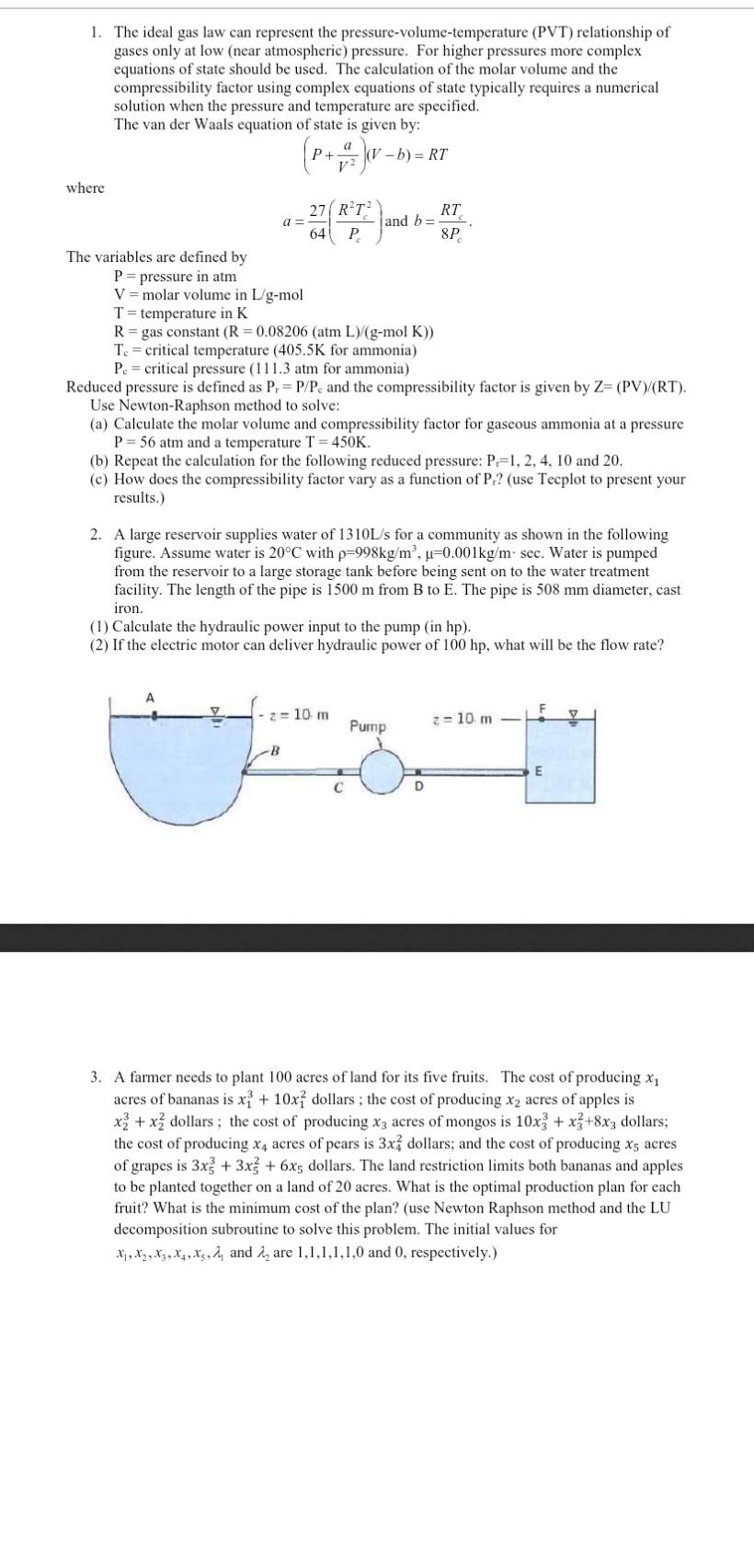
1. The ideal gas law can represent the

Fluids, Free Full-Text

Solved Problem 2. ( 30 points) The ideal gas law can

Solved Problem 2. ( 30 points) The ideal gas law can

012 IfZ is a compressibility factor, van der Waals equation low pressure can be written as: [2014] RT I-끔 (C) Z-I+ Z=1+ (B) Ζ=I.RT (D) Z=l- _ pb VRT







