Solved The compressibility factor, Z, can be thought of as a

Answer to Solved The compressibility factor, Z, can be thought of as a

Thermodynamic Properties Property Table w Property Table -- from direct measurement w Equation of State w Equation of State -- any equations that relates. - ppt download

For compressibility factor, Z, which of the following is /are correct?

Comparison of the compressibility factor Z for methane at 300K computed

c) When Z gt 1 , real gases are difficult to compress .
If Z is a compressibility factor, van der Waals' equation at low pressure can be written as - Sarthaks eConnect

Write the expression for the compressibility factor (Z) for one mole of a gas. Write the value of Z for an
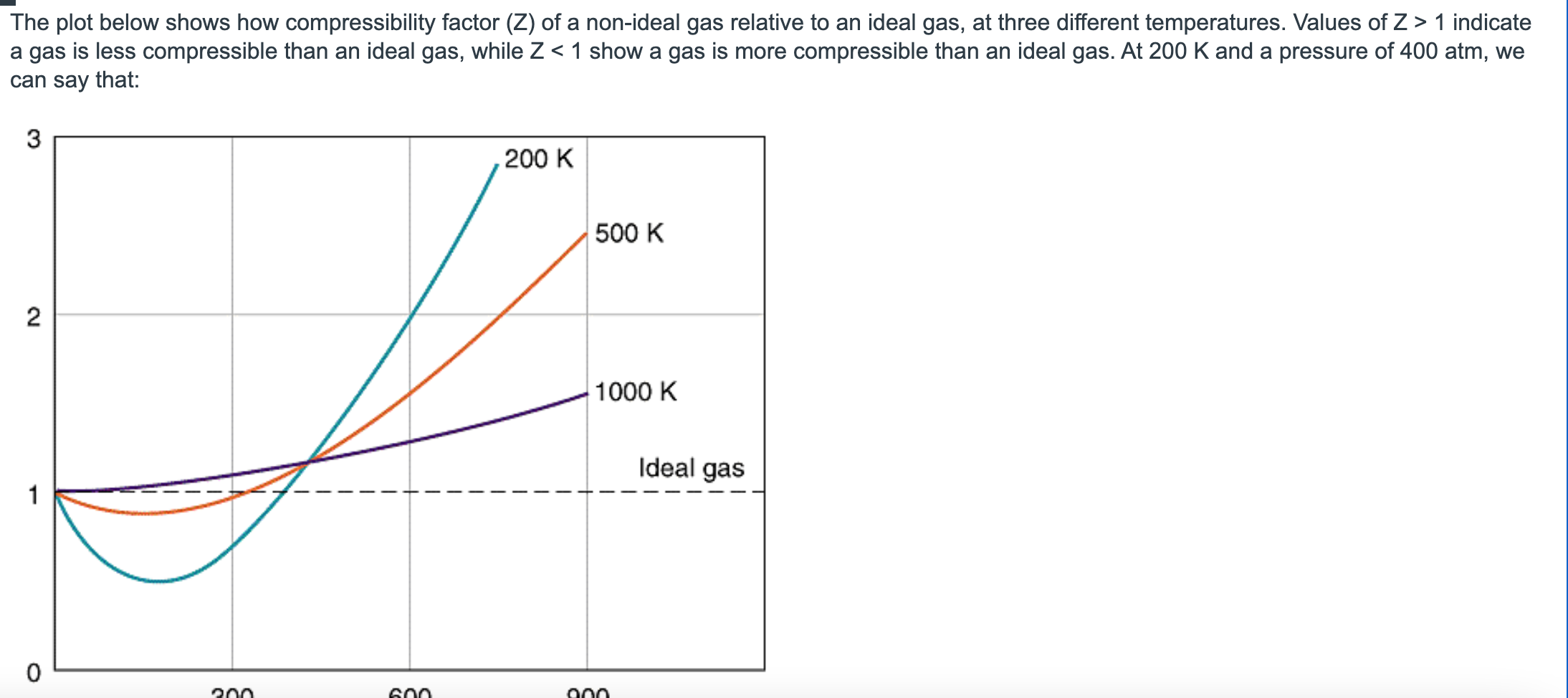
Solved The plot below shows how compressibility factor (Z)

If `Z` is a compressibility factor, van der Waals' equation at low pressure can be written as

Real Gas Behavior The Compression Factor (Z) [Example #2]
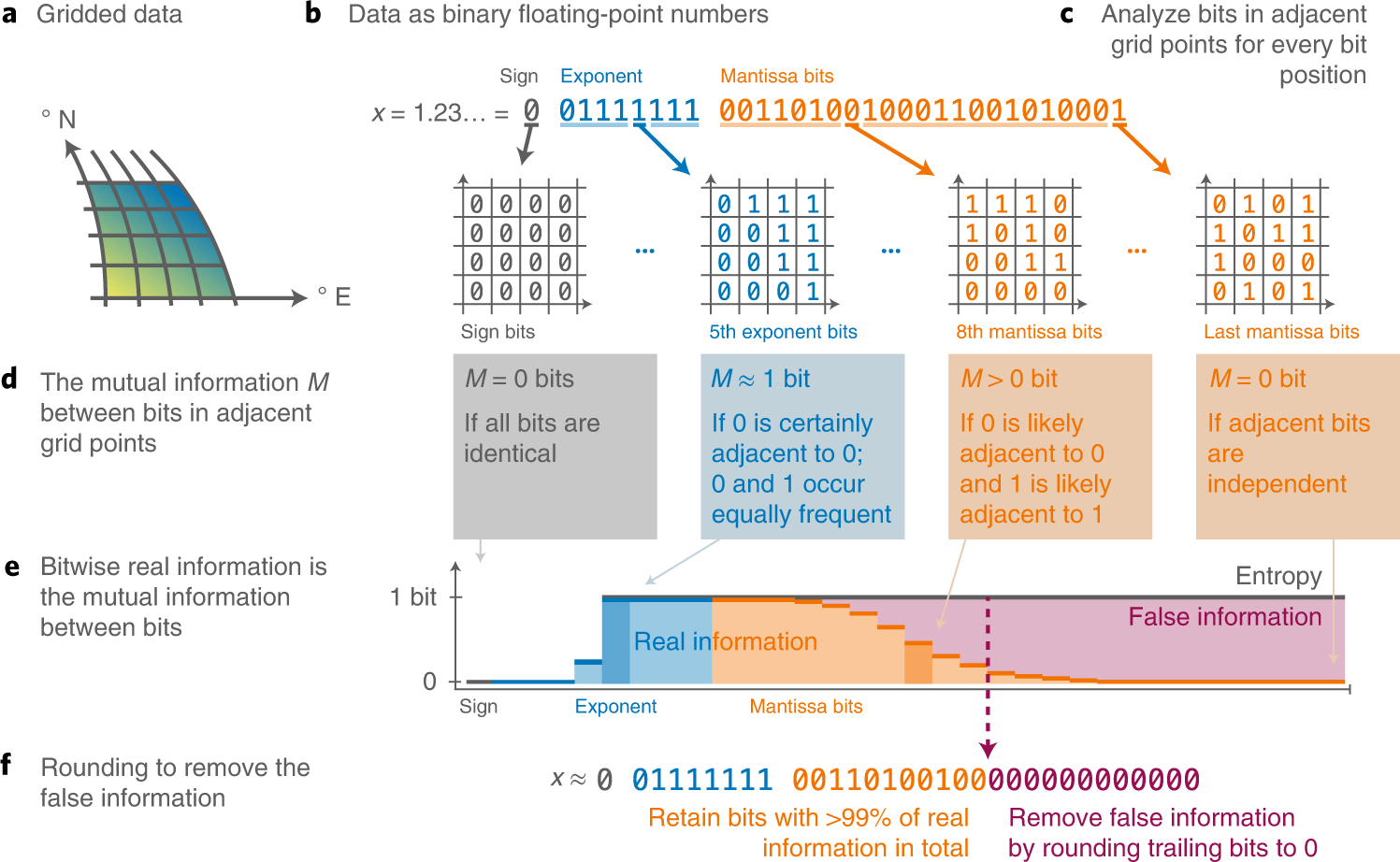
Compressing atmospheric data into its real information content







