In the following compressibility factor (Z) vs. pressure graph 300

Click here:point_up_2:to get an answer to your question :writing_hand:in the following compressibility factor z vs pressure graph at 300 k the compressibility of
Click here👆to get an answer to your question ✍️ In the following compressibility factor -Z- vs- pressure graph 300 K- the compressibility of CH-4- pressure - 200 bar deviates from ideal behaviour becauseThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is than its molar volume in the ideal stateThe molar volume of CH-4- is same as that in its ideal stateIntermolecular interactions between CH-4- molecules decreases

Energies, Free Full-Text
47. In the following compressibility factor (Z) vs pressure graph 300 K, the compressibility factor of CH4 pressures < 200 bar deviates from ideal behavior because

Compressibility Factor Z Important Concepts and Tips for JEE Main

If the slope of 'Z' (compressibility factor) vs. 'p' curve is constant `(slope=(pi)/(492.6)atm^
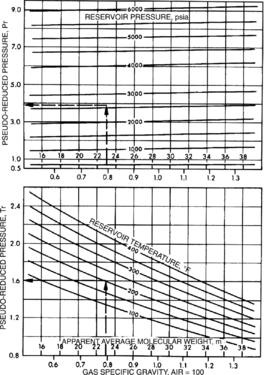
Static gas pressure gradient estimation - AAPG Wiki

Gas compressibility factor Z: Ideal gas vs Real gas

Improved description of the liquid phase properties of Methane: density, enthalpy, plus saturated vapor compressibility factor
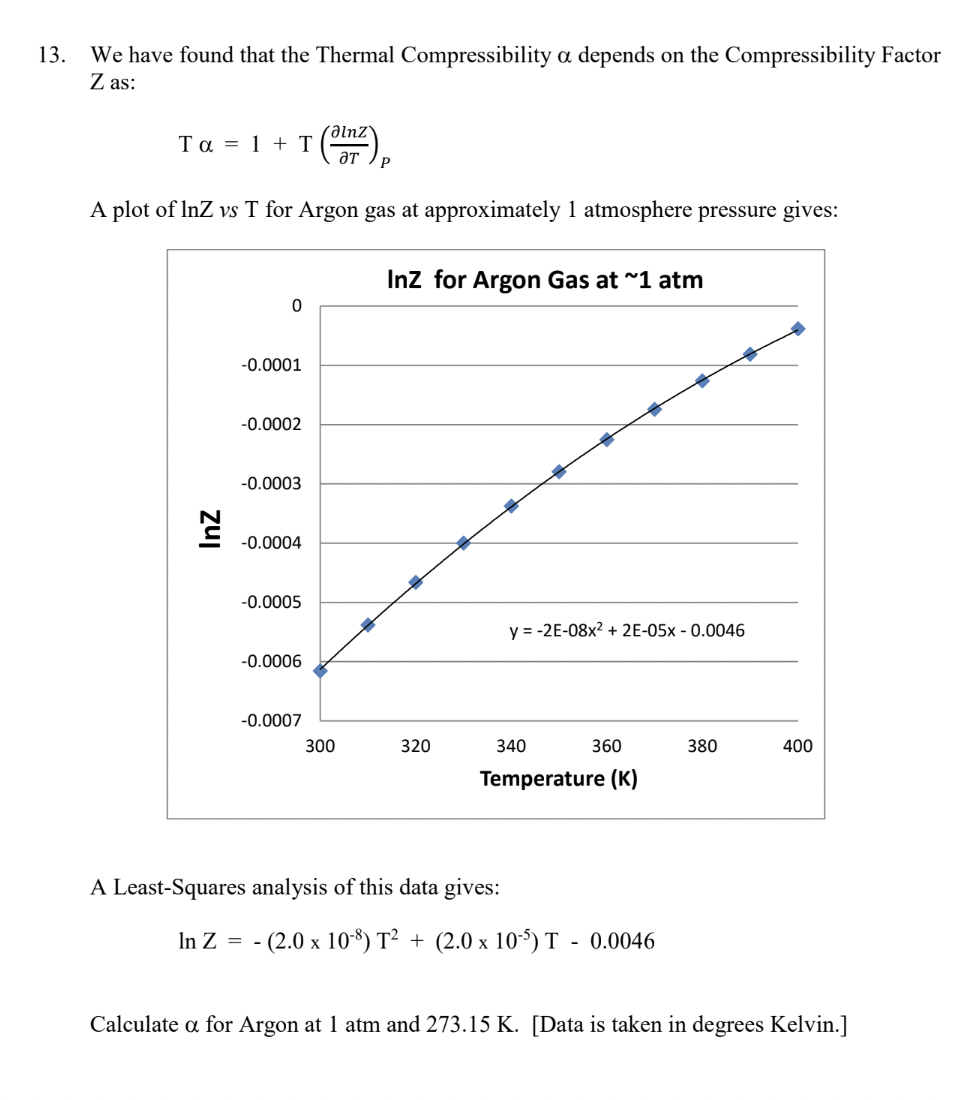
Solved 13. We have found that the Thermal Compressibility α
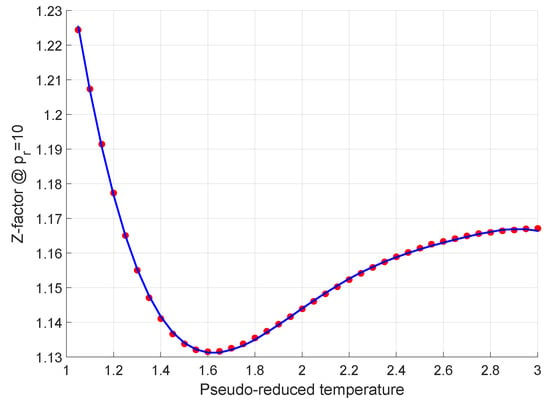
Energies, Free Full-Text
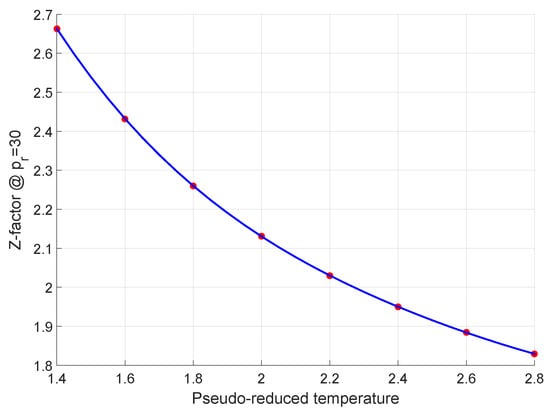
Energies, Free Full-Text

Compressibility factor of water vapor along its saturation curve. Error
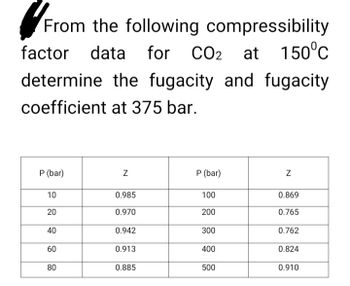
Answered: From the following compressibility…
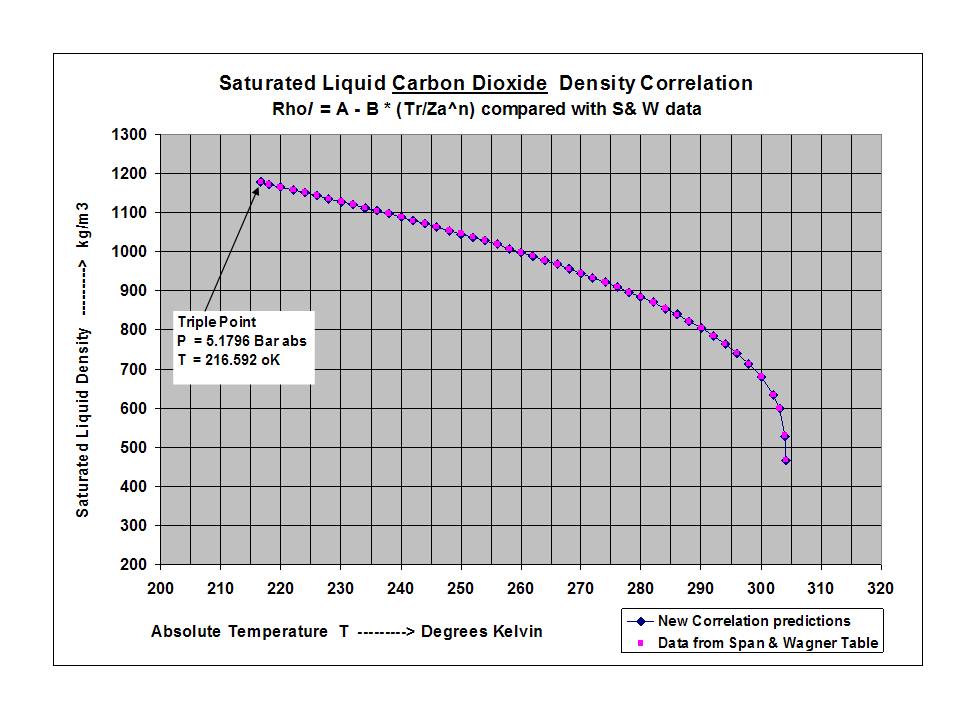
New compact Equations for the Compressibility Factor Z and Density of Liquid and Vapor Carbon Dioxide







