The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n

The given graph represents the variation of Z (compressibility factor = \\[\\dfrac{{PV}}{{nRT}}\\] ) versus P, for three real gases A, B and C. Identify the only incorrect statement.\n \n \n \n \n A.For the gas A, a=0 and its dependence

Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with
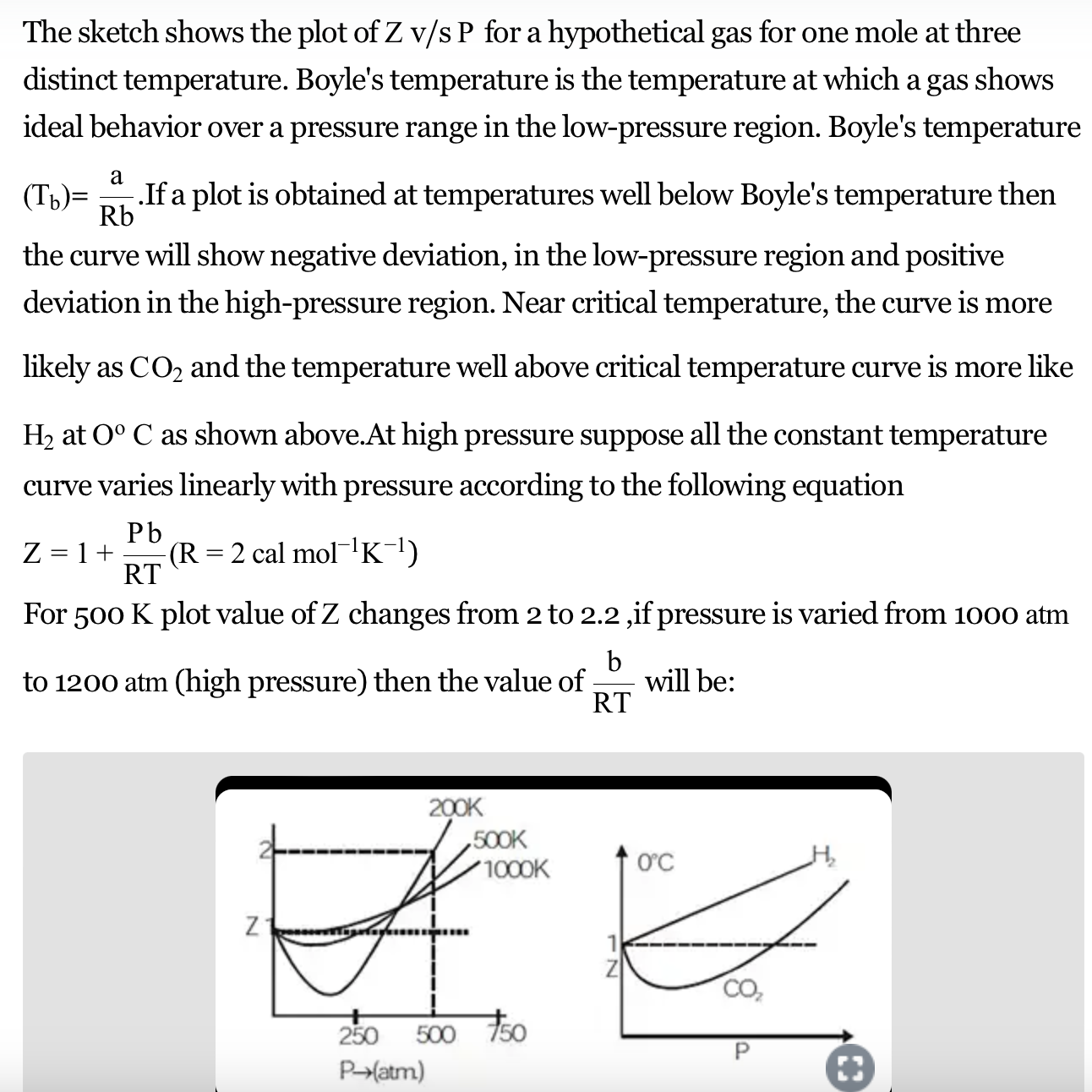
Solved The graph of compressibility factor (Z)v/sP for 1 mol

the given graph represents the variation of Z (compressibility factor =dfrac {PV}{nRT}) versus P, three real gases A, B and C. Identify the only correct statement.For the gas A, a = 0 and

Compressibility factor (Z=(PV)/(nRT)) is plotted against pressure

The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `
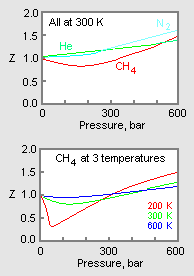
Compressibility factor (gases) - Knowino

The given graph represents the variations of compressibility factor `Z=PV// nRT` vs `
stackoverflowresearcher/archimedes.txt at master · MichalPaszkiewicz/stackoverflowresearcher · GitHub

Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with

stackoverflowresearcher/archimedes.txt at master · MichalPaszkiewicz/stackoverflowresearcher · GitHub

Compressibility Factor Z Important Concepts and Tips for JEE Main

For the gas C which is a typical real gas for which neither a nor b =0

4.2: Real Gases (Deviations From Ideal Behavior) - Chemistry LibreTexts

Thermodynamics - Test 1 Problem 5 - Ideal Gas Equation with







